Nebraska Pediatric Clinical Trials Unit
The Nebraska Pediatric Clinical Trials Unit was established in 2016 as part of the IDeA States Pediatric Network. These 18 states were awarded funding from the National Institutes of Health to participate in the Environmental influences on Child Health Outcomes program.
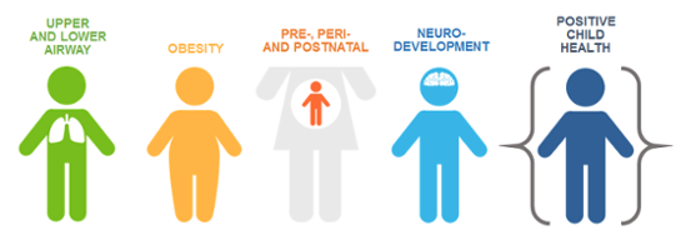
The outcomes program, known as ECHO, has a mission “to enhance the health of children for generations to come.” There are five main pediatric focus areas that were chosen based on their ability to dramatically impact public health. Several clinical trials relating to these areas are in progress, and more are in development and coming soon!
Learn more about the ECHO Program and the Institutional Development Award States Pediatric Network.
The pediatric clinical trials unit is supported by the National Institutes of Health Office of the Director, Grant Number 2UG1OD024953-03.
The Institutional Development Award program was established by the National Institutes of Health in order to create a more level playing field with regards to federal biomedical research funding. This program supports faculty development and research infrastructure enhancement at institutions in 23 states and Puerto Rico and is intended to include rural and underrepresented communities in research initiatives.
As a part of the Environmental influences on Child Health Outcomes program, the NIH utilized existing IDeA resources to create the IDeA States Pediatric Network (ISPN), awarding funding to establish pediatric research centers in states that are not generally well-represented in clinical research. The Nebraska Pediatric Clinical Trials Unit was established in 2016 as part of this project.
The Nebraska Pediatric Clinical Trials Unit is fortunate to work with the pediatricians and pediatric specialists at the University of Nebraska Medical Center and Children’s Nebraska. Access to their research infrastructures and clinical expertise provides us with a unique opportunity to reach Nebraska's rural and underserved communities and makes us well-suited to helping to fill the gap in pediatric clinical trials. This creates a unique opportunity for us to act as a central home for pediatric clinical research across the state.
The pediatric clinical trials unit is supported by the National Institutes of Health Office of the Director, Grant Number UG1OD024953.
In keeping with the goals of the IDeA States Pediatric Network, the mission of the unit is:
1) To establish the unit as the home for pediatric clinical trials in our region. This program will support the professional development of the associated faculty, residents and personnel conducting clinical research at UNMC and Children's, while building on our existing program to conduct clinical trials research for children with a variety of conditions.
2) To provide the underserved and rural populations of Nebraska and surrounding states access to state-of-the-art clinical trials. Our clinical outreach network has access to a wide variety of underserved populations who would benefit from inclusion in cutting-edge clinical research and would enhance the quality and applicability of data generated for pediatric research as a whole.
Contact Us
Nebraska Pediatric Clinical Trials Unit
985456 Nebraska Medical Center
Omaha, NE 68198-5456
Phone: (402) 689-8701
Email

