Assistant Professor
 |
Department of Genetics, Cell Biology and Anatomy |
Education:
Postdoc - Epithelial Cell Biology, Stowers Institute, USA (2022)
PhD - Cell Biology & Biophysics, ETH Zurich, Switzerland (2015)
|
Research: |
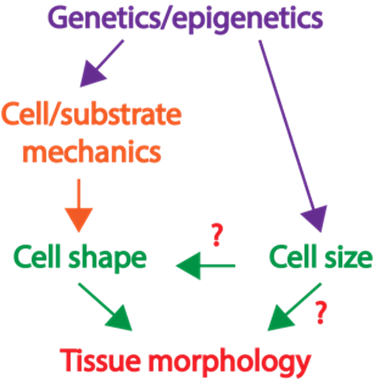
packing is largely unknown. |
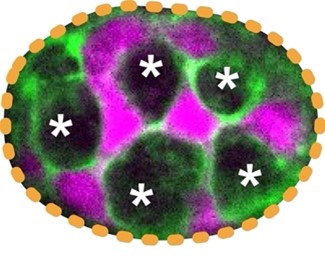
| Project 1: Determine how the large Paneth cells and small Lgr5+ stem cells at the base of the intestinal crypt domain maintain a checkerboard-like order. How animal cells of diverse shapes and sizes pack to form functional tissue is a fundamental biological question. Nevertheless, the current understanding of how tissues organize is mostly from examining the adhesive and tensile properties of similarly shaped cells. Previously, we discovered that discrepancies in cell size induce cell junctions to rearrange and cause stereotypical cellular reorganization. In our laboratory, we will dissect the principles of how, despite the rapid turnover, a checkerboard-like arrangement is maintained by the intestinal stem and support cells (Fig. 2). |
|
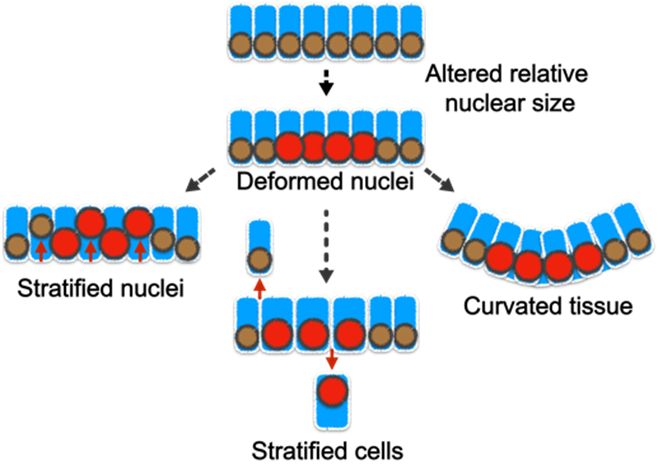
| Project 2: The tissue-level consequences of shifting the nuclear-cytoplasm volume ratio of uniform enterocytes in the intestinal villus Although cells of the same type have remarkably uniform morphology, tumor cells are typically pleomorphic and can exhibit abnormal variations in the size of cells and nuclei. Nevertheless, the influence of such variance in disease progression is not well understood. Furthermore, the tight correlation between the cell and nuclear size is frequently perturbed in diseases; but what are the pathological implications of this decoupling? Previously, we showed that abnormality in cellular size can have a profound impact on how cell pack and tissue organize. In our laboratory, we will determine how the organization of small intestinal villi in mice can be altered by abnormal cell and nuclear shape (Fig. 3).Our approach to addressing these questions is highly interdisciplinary. We integrate confocal imaging with biophysics and sophisticated genetic manipulation to quantitatively distinguish the influence of cell mechanics, shape, and size in tissue packing and homeostasis. We leverage a wide range of models including traditional 2D culture, 3D organoids, and mouse intestinal tissue. Over the long term, we anticipate that our study will extend the understanding of how cells self-assemble into tissues with stereotypical forms and reveal how functional tissue architecture is perturbed in disease. |
|
To apply, please email a cover letter, CV, and two reference letters to Dr. Subu Ramanathan.
Publications listed in PubMed
Publications:
- Cell size pleomorphism drives aberrant clone dispersal in proliferating epithelia (2019). Ramanathan S. P., M. Krajnc, and M. C. Gibson. Developmental Cell. 2019. 51 (1):49-61. (Reviewed in ‘Epithelial Morphogenesis: Size Matters’ by Barry J. Thompson. Developmental Cell (2019), 51, (1): 2-3. Featured on the cover page of Developmental Cell on October 7, 2019.)
- Cdk1-Dependent Mitotic Enrichment of Cortical Myosin II Promotes Cell Rounding Against Confinement (2015). Ramanathan S. P., J. Helenius, M. P. Stewart, C. J. Cattin, A.A. Hyman, and D. J. Muller. Nature Cell Biology. 2015. 17 (2):148-159. (Featured on the cover page of Nature Reviews Physics on November 01, 2018.)
- Assay for Characterizing the Recovery of Vertebrate Cells for Adhesion Measurements by Single-Cell Force Spectroscopy (2014). Schubert, R., N. Strohmeyer, M. Bharadwaj, S. P. Ramanathan, M. Krieg, C. M. Franz, and D. J. Muller. Federation of European Biochemical Societies Letters. 2014. 588 (19):3639-3648.
- Hydrostatic Pressure and the Actomyosin Cortex Drive Mitotic Cell Rounding (2011). Stewart, M.P., J. Helenius, Y. Toyoda, S. P. Ramanathan, D.J. Muller, and A.A. Hyman. Nature 469 (7329):226-230.
- Type III Restriction Enzymes Cleave DNA by Long-Range Interaction Between Sites in Both Head-to-Head and Tail-to-Tail Inverted Repeat (2010). Van Aelst, K., J. Tóth, S. P. Ramanathan, F.W. Schwarz, R. Seidel, and M.D. Szczelkun. Proceedings of the National Academy of Sciences. 107(20):9123-8.
- Type III Restriction Enzymes Communicate in 1D Without Looping Between Their Target Sites (2009).
Ramanathan, S. P., K. van Aelst, A. Sears, L.J. Peakman, F.M. Diffin, M.D. Szczelkun, and R. Seidel. Proceedings of the National Academy of Sciences, 106(6):1748-1753.

