Single IRB is a model of IRB review that is intended to streamline and reduce administrative burden on IRBs reviewing multisite studies. In a single IRB model, one IRB serves as the reviewing IRB or “IRB of Record” and the participating site (pSite) IRBs serve as relying IRBs. The responsibilities of each IRB are outlined in a document called a reliance agreement.
For more information on the history of single IRB (sIRB) review and sIRB review at UNMC, click each section below:
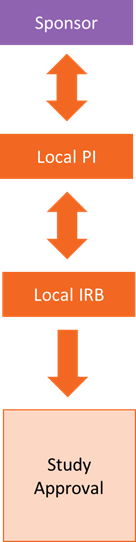
Below is a model of single site research, or research that is only taking place at one site.
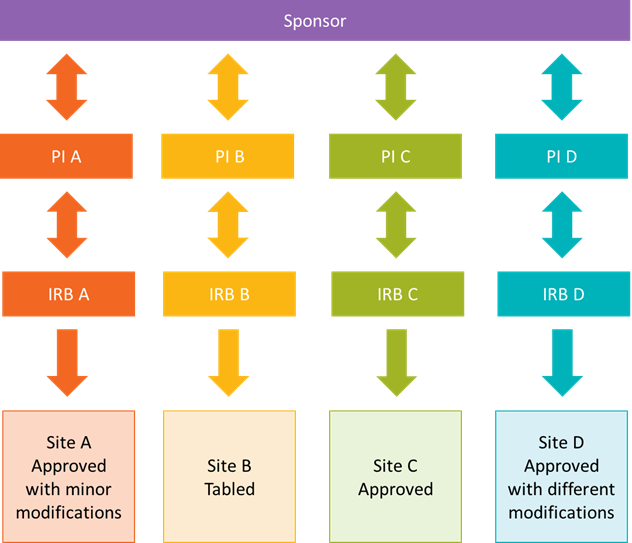
Historically, multisite research was overseen by multiple independent IRBs. This often led to differences in IRB review and outcomes across sites.
Below is a model of historical multisite research, or research that took place at more than one site.
The requirement for single IRB review originated from the NIH single IRB policy and the Revised Common Rule.
From the NIH:
"expectation that all [domestic] sites participating in multi-site studies involving non-exempt human subjects research funded by the National Institutes of Health will use a single Institutional Review Board (sIRB)" (NOT-OD-16-094, June 2016)
♦ effective date January 25, 2018
For more information on the NIH Single IRB Policy visit: https://grants.nih.gov/policy/humansubjects/single-irb-policy-multi-site-research.htm.
For Guidance on exceptions to the NIH Single IRB Policy, visit: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-003.html
From the Revised Common Rule:
"Any institution located in the United States that is engaged in cooperative research must rely upon approval by a single IRB …" (§_.114(b)(1))
♦ effective date January 20, 2020
For more information on the Revised Common Rule's Single IRB Requirement, visit: https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46#46.114.
For a list of Common Rule Departments and Agencies, visit: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html
For information on Common Rule Exception Determinations, visit: https://www.hhs.gov/ohrp/regulations-and-policy/single-irb-requirement/index.html#exceptions
In short, this means that studies that are federally funded and taking place at more than one site are now required to use a single IRB as the IRB of record. There are many circumstances where it is not clear if a specific study requires single IRB review or if an exception may apply. In these cases, please email sirb@unmc.edu for assistance at the earliest opportunity.
Note: Participating site IRBs are still involved in the single IRB process and have their own local requirements, but the responsibility for the regulatory component of review is ceded to the single IRB. Each IRB’s responsibilities are outlined in a document called a Reliance Agreement. More information on reliance can be found in the sIRB Reliance section below.
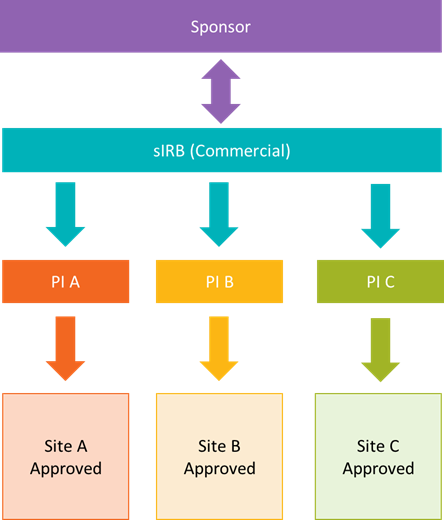
Some groups were already utilizing a single IRB model prior to the requirement (e.g., commercial IRBs).
Below is a simplified model of how some commercial IRBs handle multisite research. Many will interact directly with the sponsor, perform IRB review activities, and then push approvals to all sites simultaneously.
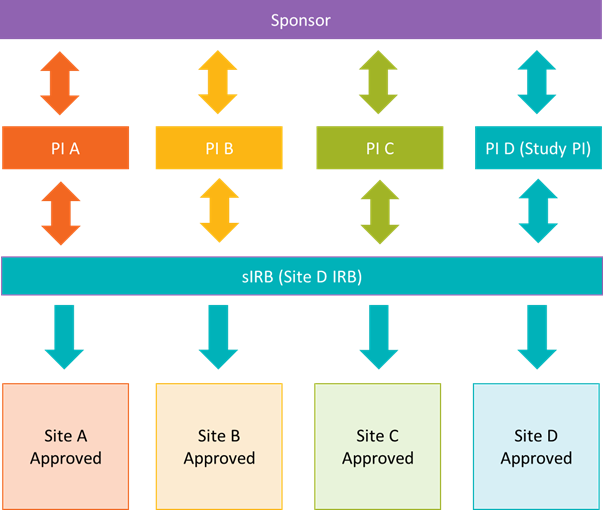
Below is another model of how a single IRB may handle multisite research. In this example, the sponsor works with each site PI and study team independently. Each site submits their own application to the single IRB for review and receives separate approvals.
Single IRB Review at UNMC is broken out into two main parts. First, the overall study and lead site-specific requirements are reviewed. After overall study and lead site approval, participating sites (pSites) are onboarded and approved on a rolling basis.
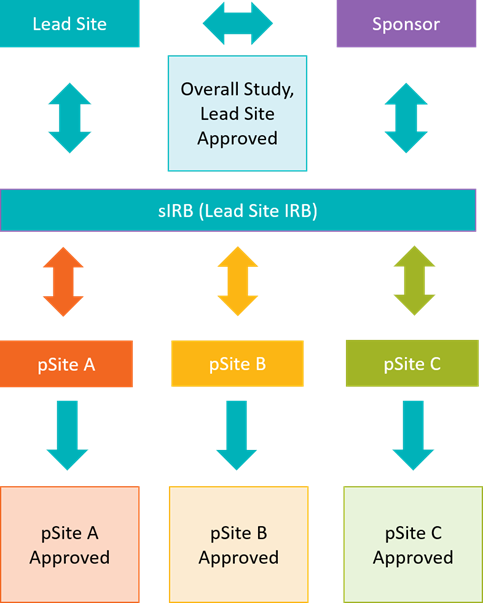
Note: pSite IRB processes are not included in the above models. Please contact your pSite IRB directly with questions.

