Sutapa Ray Lab

Research Interest
The overall goal of Dr. Sutapa Ray's laboratory is to delineate the functional role of Interleukin-6 (IL-6)-Janus kinases (JAKs) and the downstream Signal Transducers and Activators of Transcription-3 (STAT3) signaling pathway in pediatric brain cancer Medulloblastoma (MB). The IL-6/JAK/STAT3 pathway is aberrantly hyperactivated in many types of cancer, and such activation is generally associated with a poor clinical prognosis. STAT3 transcription factor drive the proliferation, survival, invasiveness, and metastasis of tumor cells, while strongly suppressing the chemosensitivity and antitumour immune response.
STAT3 acts as an oncogene in MB pathogenesis: Dr. Ray's lab had showed that activated STAT3 promotes MB cell growth and survival by inducing expression of several downstream targets, including the non-coding oncomiR, microRNA-21 (miR-21). MiR-21, in turn, represses protein inhibitor of activated STAT3 (PIAS3) expression, a STAT3 negative regulator, leading to persistent STAT3 activation and chemo-resistance in the most aggressive MYC-amplified MB. Given that constitutively activated STAT3 plays a critical role in multiple pro-survival and oncogenic pathway, Dr. Ray's lab is specifically targeting STAT3 in MB by doxycycline induced shRNA-mediated depletion or by pharmacological inhibition with small molecule inhibitors. This study will provide the proof of concept that STAT3 has a direct role in MB pathogenesis and chemoresistance. STAT3 inhibitors thus can be used to augment treatment efficacy and reduce therapy-related toxicity in high-risk patients, improving the quality of life of childhood cancer survivors.
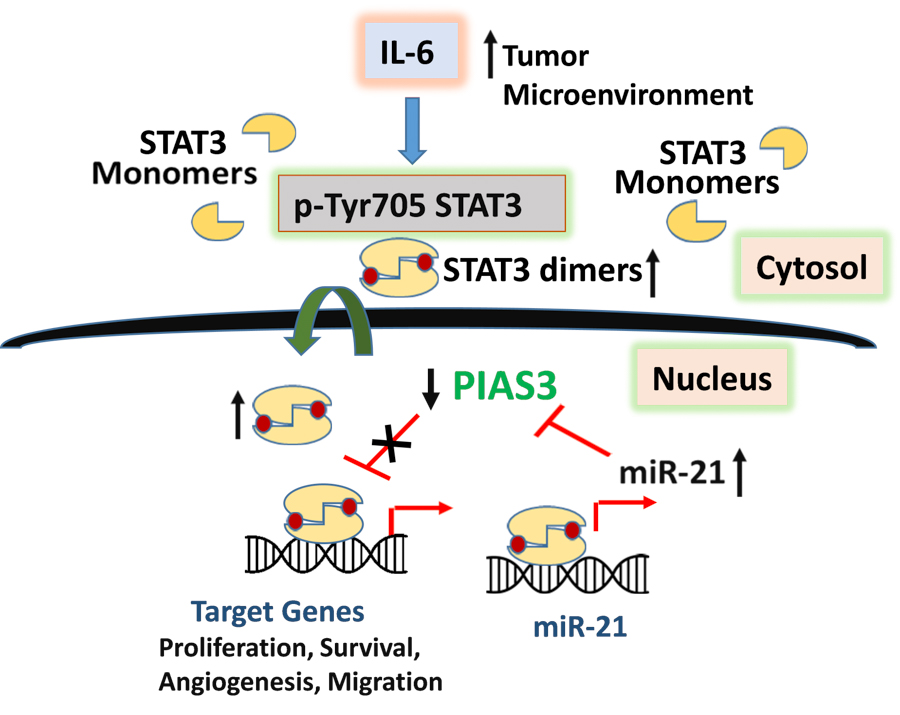
Schematic diagram of STAT3 mediated MB pathogenesis. IL6 stimulated pSTAT3 activation in MB upregulates STAT3 target genes including miR-21. MiR-21 represses PIAS3, a STAT3 negative regulator, thereby inducing persistent STAT3 activation, chemoresistance and MB pathogenesis. [Ray et al 2018; PMID 29280516]
Role of STAT3-Cyclin-dependent kinase 9 (CDK9) axis in MB: CDK9 and its regulatory cyclin partner, cyclin T1, together known as P-TEFb complex, is widely implicated in the control of transcription elongation by phosphorylating Serine 2 (Ser2) in the RNA Polymerase II (RNA Pol II) C-terminal domain (CTD). CDK9 plays a pathogenic role in various cancers and Dr. Ray's lab showed that CDK9 is highly expressed in human MB tumors and increased CDK9 expression is correlated with high-risk MB patients. Dr. Ray's lab has recently found that STAT3 forms an inducible complex with CDK9 in MB. Using RNA-Seq and ChIP-Seq assay, the lab will explore the role of CDK9 in STAT3 gene network and will identify novel target proteins responsible for inducing MB tumor initiation and progression. Further, by targeting STAT3-P-TEFb axis, Dr. Ray's lab will also test the efficacy of inhibitors in attenuating MB tumor growth in orthotopic MB mouse model.
Role of STAT3 in regulation of Program cell death ligand-1 (PD-L1) expression in MB: PD-L1 expression and regulation in pediatric MB remains unexplored. Understanding the molecular mechanisms of how PD-L1 expression on MB cells are regulated may aid in developing new drug targets and combination therapies that will reverse the tumor immune evasion and thereby elicit response to immunotherapies. Given the promise of PD-L1/PD-1-based therapies in Melanoma, NSCLC, Bladder, Ovarian cancer etc, Dr. Ray's lab aimed to characterize regulation of PD-L1 expression by STAT3 in MB and to identifying the optimal immuno-competent mouse model to evaluate the efficacy of checkpoint inhibitor therapy in vivo, e.g. PD-L1 expression, lymphocyte infiltration and T-cell activation.
Contact Information
Lied Transplant Center
11732 Nebraska Medical Center
Omaha, Nebraska 68198-7627
Academic Office/Lab
Office: LTC 11733; (402) 559 4250
Lab: LTC 11728; (402) 559 4241
(402) 559 4208 (Fax)
Email: sutapa.ray@unmc.edu

Dr. Sutapa Ray (far left) and her lab team
Lab Features
Erin McIntyre, MS (Research Coordinator); PCRG core member
Gracey Alexander, BS (Research Technologist); PCRG core member
Role: Principal Investigator
Funding Agency: Team Jack Power 5 Neuro-Oncology Research Grant
Role: Principal Investigator
Funding Agency: Edna Ittner Pediatric Research Support Grant
Role: Principal Investigator