Current Projects
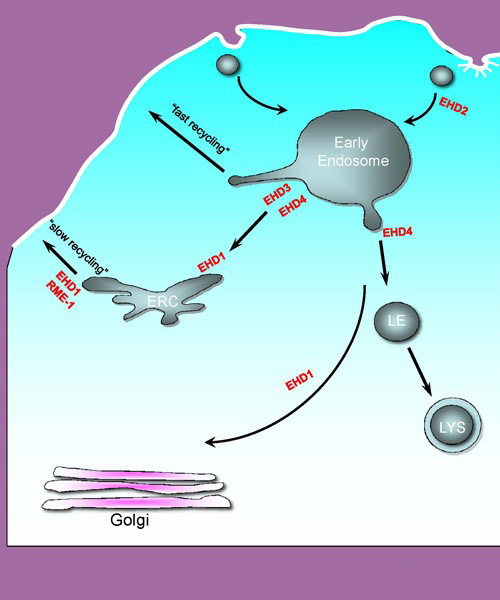
This schematic diagram illustrates some of the endocytic pathways in which receptors travel, once they have been internalized from the cell membrane, and it depicts key proteins that regulate these processes.
The Caplan laboratory is interested in understanding the basic mechanisms and pathways that control the movement of receptors, proteins and lipids from point to point within the cell—a process known as “MEMBRANE TRAFFICKING,” or “VESICULAR TRANSPORT.”
This schematic diagram illustrates some of the endocytic pathways in which receptors travel, once they have been internalized from the cell membrane, and it depicts key proteins that regulate these processes. Rab GTP-binding proteins and Eps15 homology Domain (EHD) proteins are among some of the proteins we study. Multiple studies from our lab and others have implicated these proteins in the steps needed to move proteins from organelle to organelle within the cell, or to recycle receptors back to the plasma membrane.
The internalization of cell surface molecules from the plasma membrane is a critical event for all eukaryotic cells. While many internalized molecules are degraded in the endo-lysosomal pathway, many receptors, proteins and other molecules undergo sequential rounds of recycling back to the plasma membrane. Endocytic recycling is key for the control of cell surface receptors on the plasma membrane. Since most receptors generally transduce signals only when bind ligands at the plasma membrane (PM), this means that regulation of their localization to the PM may have a critical impact on signal transduction, and cell proliferation, which is directly related to cancer. Endocytic recycling also governs the flow of nutrients into the cell, and can compensate for the loss of membrane lipids incurred during the process of internalization. Other specialized recycling functions include: synaptic vesicle recycling, iron homeostasis in liver, MHC Class II and MHC Class I molecules, transcytosis of Transferrin receptor and Immunoglobulin A (IgA) across polarized epithelial cells, and insulin-dependent glucose transport in muscle and adipose cells.
Since our laboratory follows our discoveries wherever they lead, research in the lab has expanded to studying the actin cytoskeleton and regulation of actin polymerization, the process of cytokinesis and cell division, the study of primary cilia and their biogenesis, centrosome biology, and most recently, mitochondrial dynamics and their continual fusion and fission.