Current Projects
Our research projects include:
Protein Tyrosine Phosphorylation signaling
Protein tyrosine phosphorylation by several oncogene proteins and growth factor receptors has been well documented to play a crucial role in the control of cell growth. This is a cyclical process of regulation including phosphorylation and dephosphorylation, which can be regulated at either step. To delineate this cyclical regulatory mechanism, we examine the putative function and regulation of the cellular form of human prostatic acid phosphatase (cellular PAcP), a prostate epithelium-specific protein tyrosine phosphatase. This is because cellular PAcP is decreased in prostate cancer cells and its expression can be regulated by androgens, which is a risk factor for prostate carcinogenesis. Our results show that the expression of cellular PAcP correlates with the androgen responsiveness of human prostate cancer cells. In those cells, cellular PAcP dephosphorylates HER-2/ErbB-2/neu proto-oncoprotein at specific phosphotyrosine residues. Thus, the interaction of cellular PAcP and ErbB-2 regulates androgen sensitivity of prostate cells. The role of cellular PAcP as a tumor suppressor by acting as a PTEN-functional homologue is clearly evidenced by results of molecular and cellular biology approaches. Supportively, in PAcP-knockout male mice, the prostate spontaneously develop adenocarcinomas.
Two US Patent-awarded Prostate Cancer Cell Progression Models
To investigate the molecular alteration during cancer progression, we established two US Patent-awarded prostate cancer cell progression models that recapitulate advanced cancer progression, including:
- Androgen-independent cells derived from androgen-sensitive cells recapitulating clinical tumor progression
- Neuroendocrine (NE)-like prostate cancer cells mimicking clinical androgen-deprivation therapy.
These two cell models together would allow us to elucidate the molecular mechanism of cancer progression, leading to the development of targeting therapies.
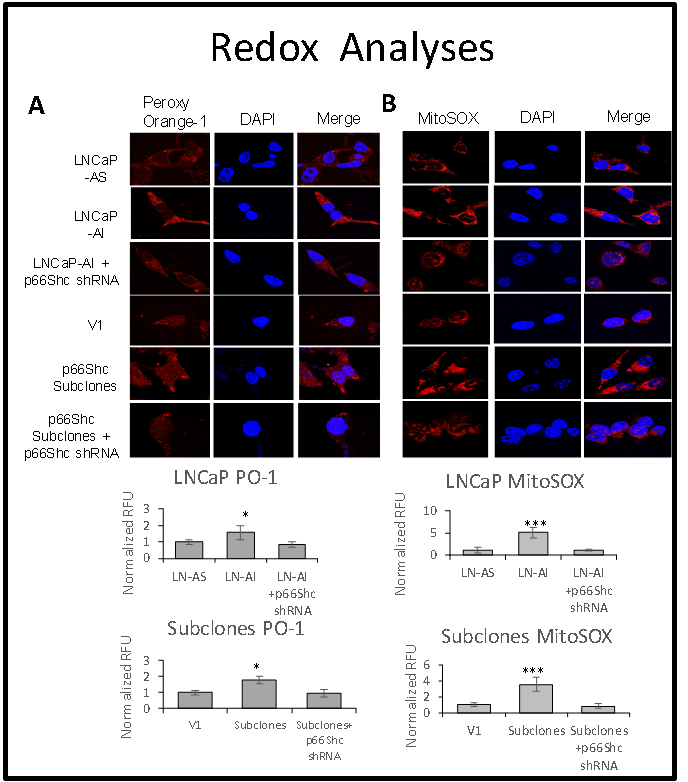
p66Shc, a pro-oxidant protein, is responsible for the elevated reactive oxygen species (ROS) level in advanced prostate cancer cells.
Redox signaling
Our data further reveal that Redox signaling plays a critical role in regulating prostate cell growth and is mediating the non-genomic androgen stimulation of cell proliferation, in part via regulating protein tyrosine phosphorylation signaling. We discovered a putative oncogenic protein, p66Shc, a pro-oxidant protein elevated in prostate cancer cells, in promoting the tumorigenicity of prostate epithelia as well as its advanced stage progression and metastasis. We investigate its mechanism, as shown in the Figure.
Small Molecule Analyses
For immediate translational applications, we pursue the identification of novel, small molecule compounds targeting to drug-resistance cancer cells. We have identified a few small molecules that are potentially applicable for targeting advanced cancer cells, including prostate cancer cells. Further investigation is on the way.
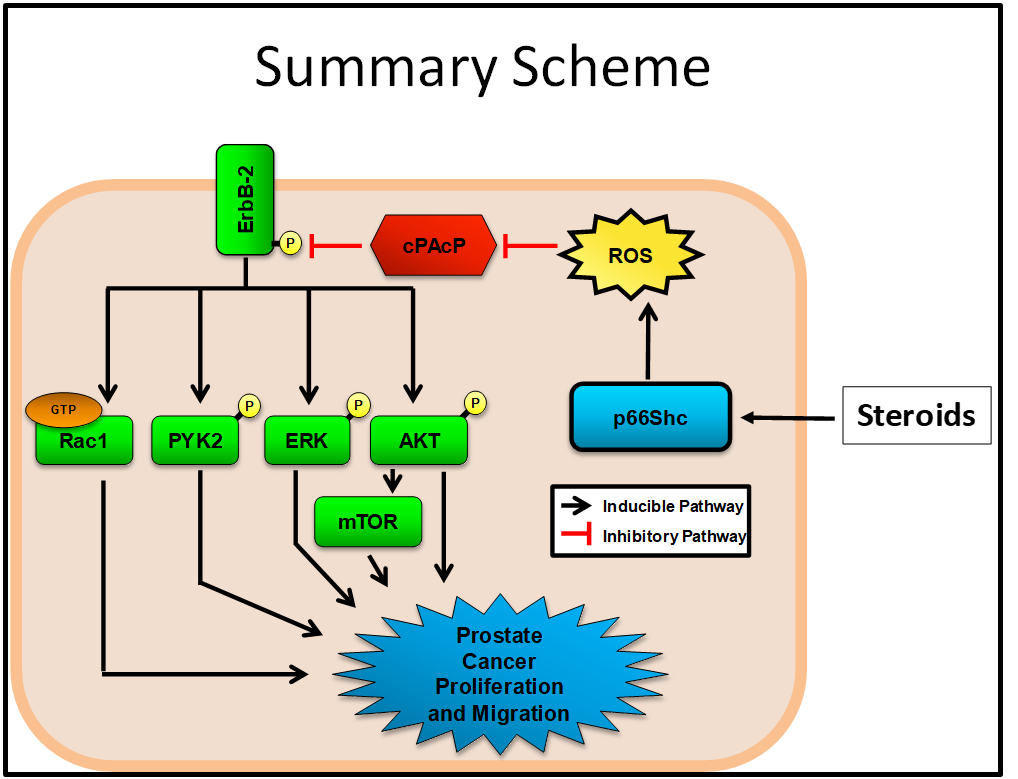
Steroid stimulation of cell proliferation is in part mediated by p66Shc-enhanced reactive oxygen species (ROS) which can turn on multi-pathways for castration-resistant prostate cancer progression.
In summary, we are elucidating the molecular mechanism of the cross-talk including steroid hormone Androgen, Tyrosine phosphorylation and Redox signaling which mediate cell growth regulation and tumorigenesis. The scheme represents a brief description of our current integration project that Androgen-enhanced ROS signaling plays a role in prostate cancer progression and metastasis.
For translational applications, our approaches include various molecular and cellular approaches, e.g., global proteomic and phospho-proteomic array analyses, in conjunction with xenograft animal models and clinical archival specimens. We will obtain information necessary for a better understanding in various aspects of androgen effects on prostate cancer biology. For immediate clinical applications, we investigate the development of novel therapeutic approaches including novel chemo-reagents applicable for the cases refractory to hormonal therapy of the advanced cancer.