Stopping respiratory illnesses before they start through new delivery systems and by such mundane work as analyzing a city’s wastewater were among the early highlights at this year’s IDWeek meeting.
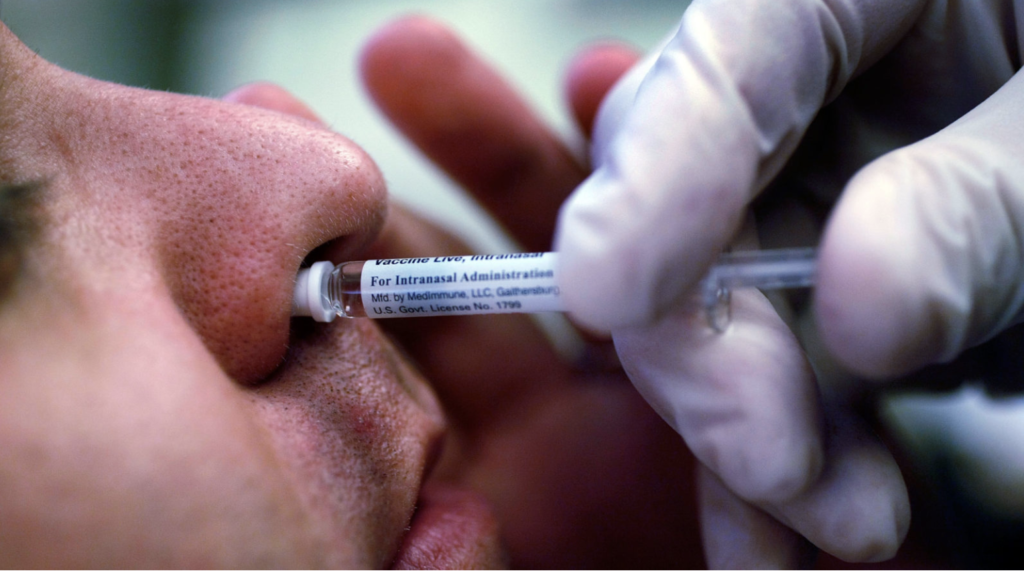
Intranasal Vaccine Shows Promise
At a press briefing, researchers suggested that those who dislike the idea of having to get poked by needles to prevent infection with SARS-CoV-2 may be able to sniff a vaccine instead.
Johanna Kaufmann, PhD, executive vice president for oncology and immunology at Codagenix, based in Cambridge, Massachusetts, reported that in a phase I study, two doses of a live-attenuated COVID-19 vaccine candidate known as CoviLiv produced a broad humoral and cellular immune response when administered intranasally.
The first-in-human trial was a primary vaccination series study conducted among healthy adults prior to the development of the mRNA vaccines that are now approved for broad public use, Kaufmann noted.
All participants exceeded a twofold increase in spike-specific IgG, with a geometric mean fold rise of 19.5 by day 57. Neutralizing antibodies at this time point were induced 2.6-fold with the microneutralization assay and 4.9-fold using the pseudovirus neutralization assays. On day 36 post-vaccination, interferon-gamma response by ELISpot increased 4.5-fold in the two-dose cohort and 2.5-fold in the one-dose cohort.