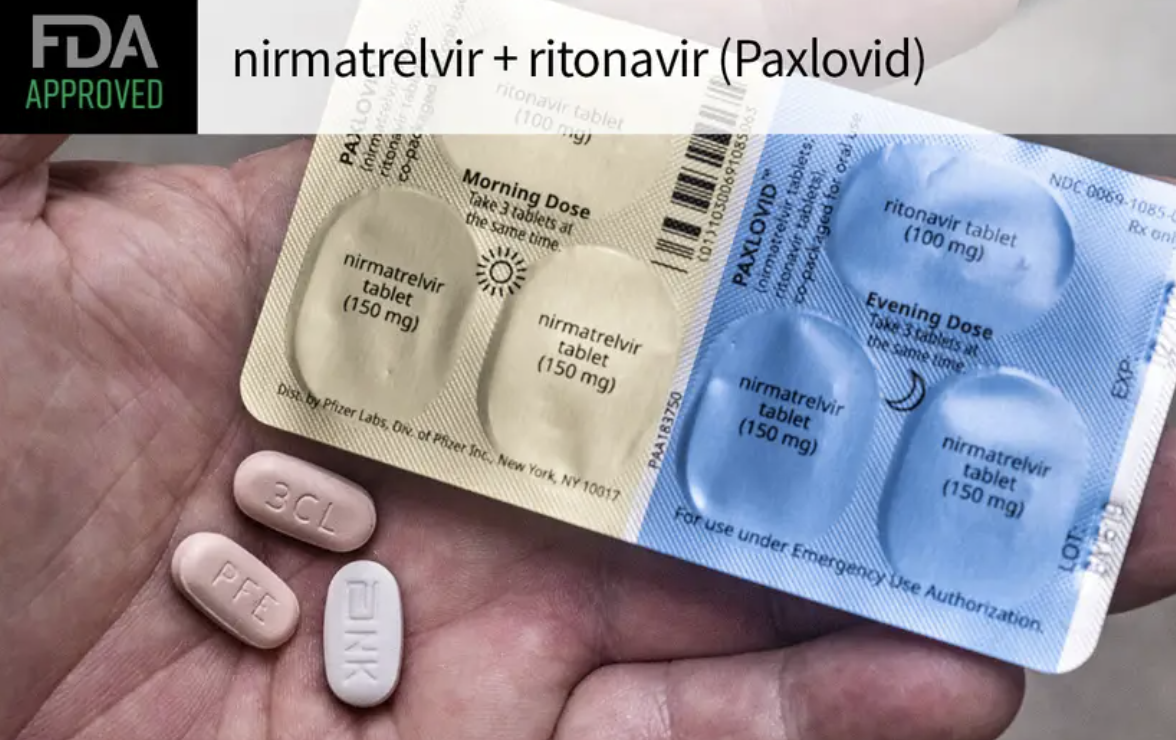
The FDA has granted full approval to nirmatrelvir-ritonavir (Paxlovid) for treating adult outpatients with mild to moderate COVID-19 who are at risk for severe disease, the agency announced on Thursday.
“Today’s approval demonstrates that Paxlovid has met the agency’s rigorous standards for safety and effectiveness, and that it remains an important treatment option for people at high risk for progression to severe COVID-19, including those with prior immunity,” Patrizia Cavazzoni, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a statement.
Approval of the antiviral — which helps ward off hospitalization and death in high-risk patients — follows an endorsement from the agency’s Antimicrobial Drugs Advisory Committee and was largely anticipated, despite some concerns about reboundopens in a new tab or window cases and a host of drug-drug interaction, which garnered the drug a boxed warning on its label and fact sheet for healthcare providers.
“Prescribers should review all medications taken by the patient to assess for potential drug-drug interactions and determine if other medicines that a patient may be taking require a dose adjustment, interruption and/or additional monitoring,” according to the FDA. “Prescribers should consider the benefit of Paxlovid treatment in reducing hospitalization and death, and whether the risk of potential drug-drug interactions for an individual patient can be appropriately managed.”
Nirmatrelvir-ritonavir was a critical component of President Biden’s test-to-treat strategy during the pandemic. Given that the antiviral is indicated for use within 5 days of symptom onset, the program aimed to get it into the hands of patients testing positive quickly, and even allowed pharmacists to prescribe the drug directly to patients.