Ex-vivo Stem Cell Approach to Retinal Degeneration
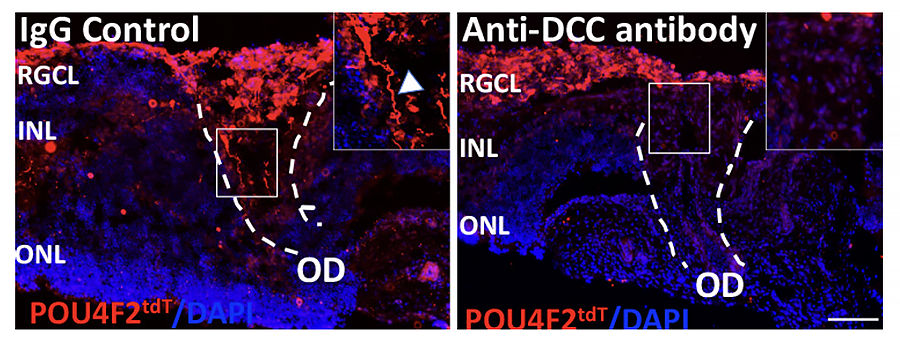
Transplanted human RGCs read the chemotropic cues in the developing rat retina to align their axons centripetally toward optic disc (OD) and that their entry into the OD is regulated by Netrin-1/DCC interactions as observed previously for rodent RGC axons.
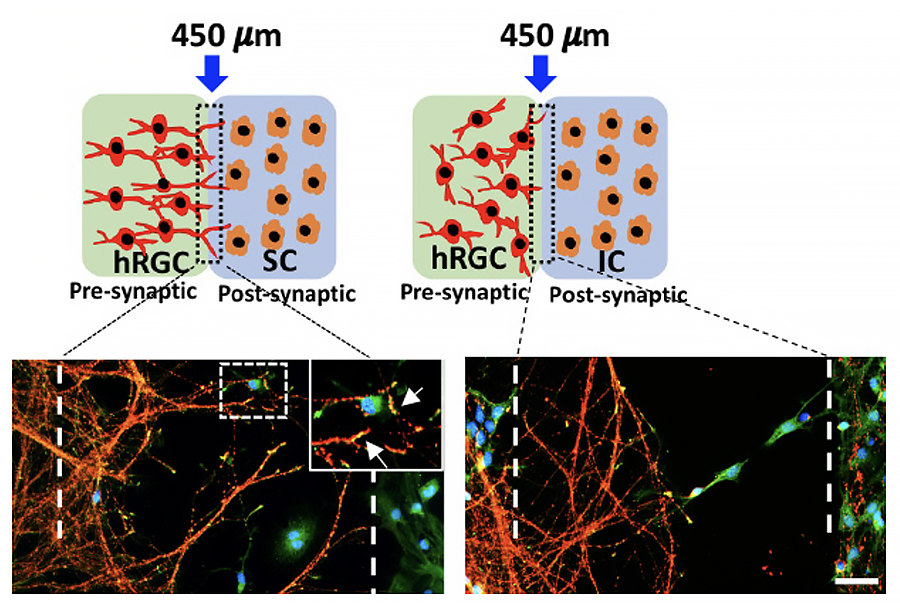
Transplantation of stem cell-derived photoreceptors and RGCs hold promise for replacing degenerating neurons in retinitis pigmentosa (RP)/age-related macular degeneration (AMD) and glaucoma, respectively. This approach was first tested in our lab where stem cell-derived retinal progenitor cells (RPCs) transplanted in the subretinal space survived and expressed photoreceptor specific marker, rhodopsin (Chacko et al., 2000, BBRC). We are currently examining the transplantation potential of pluripotent stem cell-derived human RGCs given the challenge it presents in terms of RGCs not only incorporating within the retina but also elaborating guidable axons that can navigate their way out of the retina to reach the central targets and make synaptic connections. This requirement is essential for the recovery of vision. Toward this end we are examining the potential of pluripotent stem cell-derived human RGCs to recognize and read evolutionarily conserved chemotropic guidance cues in the rodent retina to determine the clinical relevance of the approach. We have demonstrated that the transplanted human RGCs read the chemotropic cues in the developing rat retina to align their axons centripetally toward optic disc (OD) and that their entry into the OD is regulated by Netrin-1/DCC interactions as observed previously for rodent RGC axons (Subramani et al., 2023, Stem Cells)(upper panel). These observations suggests that the human RGC axons have the whereabout at least for completing the first leg of their journey, i.e., finding their way to the OD upon transplantation. In addition, we have demonstrated that hESCPOU4F2-tdT-derived hRGCs are capable of discriminating between specific (superior colliculus; SC) and non-specific (inferior colliculus; IC) central target cells and form synaptic connections (Subramani et al., 2023, Front. Cell. Dev. Biol.) (lower panel).