Endogenous Stem Cells and Retinal Regeneration
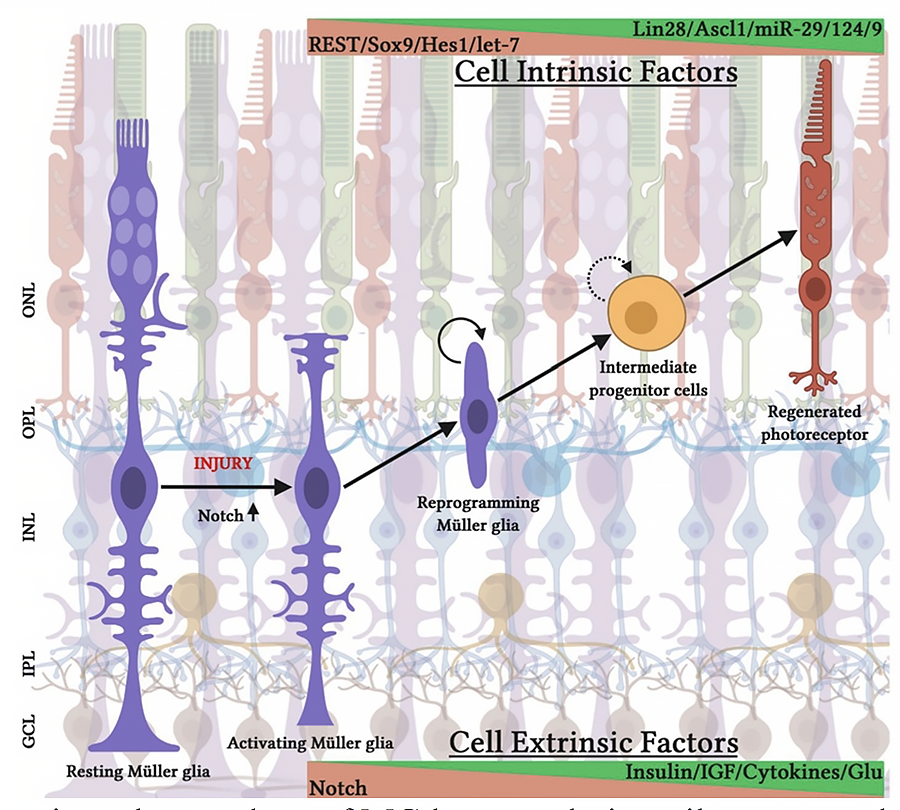
The regulation of Muller glia-the dormant stem cells in the adult retina-by intrinsic (Lin28a) and extrinsic (Notch signaling) factors to generate retinal neurons.
The discovery that the adult brain harbors stem cells that sustain neurogenesis throughout life has opened the possibility of treating degenerative changes from within by recruiting endogenous progenitors. This concept of in vivo cell therapy appeared remote for retinal degeneration because active neurogenesis has not been detected in the adult mammalian retina. However, neurogenic changes have been observed in injured adult retina, and the source of injury-induced neurogenesis is traced to Müller glia (MG), the sole glia generated by retinal progenitors. This and our observations that a subset of MG have evolutionarily conserved neural stem cell properties (Das at al., 2007, Dev. Biol.), posit these cells as a valid target for therapeutic regeneration in degenerative blinding diseases from within, circumventing the ex-vivo stem cell approach which involves transplantation and is immunogenic (Ahmad, 2011, IOVS). Therapeutic regeneration through MG is an active area of research in our lab. In animal models of retinitis pigmentosa (RP) we demonstrated that MG could be activated by Notch and Wnt signaling to differentiate along the rod photoreceptor lineage (Del Debbio et al., 2010, PLos ONE). Currently, we are examining the factors that constitute barriers to efficient conversion of MG along the neuronal lineage. We have identified genes (Lin28) and pathways (Notch and Wnt signaling) as potential targets for unlocking the neurogenic potential of MG through small molecules (Xia et al., 2022, Stem Cells)