Discovery of lenticular Peroxiredoxin 6 (Prdx6), a moonlighting protein, in eye lens
Using targeted inactivation of Prdx6 gene in mice, Singh and his research team have shown that Prdx6-depleted lenses and/or LECS contain elevated levels of reactive oxygen species (ROS), and bioactive TGF β1 and its inducible genes. TGFβ1 inducible genes, such as α-SM-actin and βig-h3, are implicated in the pathophysiology of cataractogenesis. These findings show that Prdx6 plays a rheostat role in regulating gene expression by controlling the ROS level. This led to development of a model system, Prdx6 knockout, for studying the role of ROS-driven oxidative stress-induced deleterious signaling in aging.
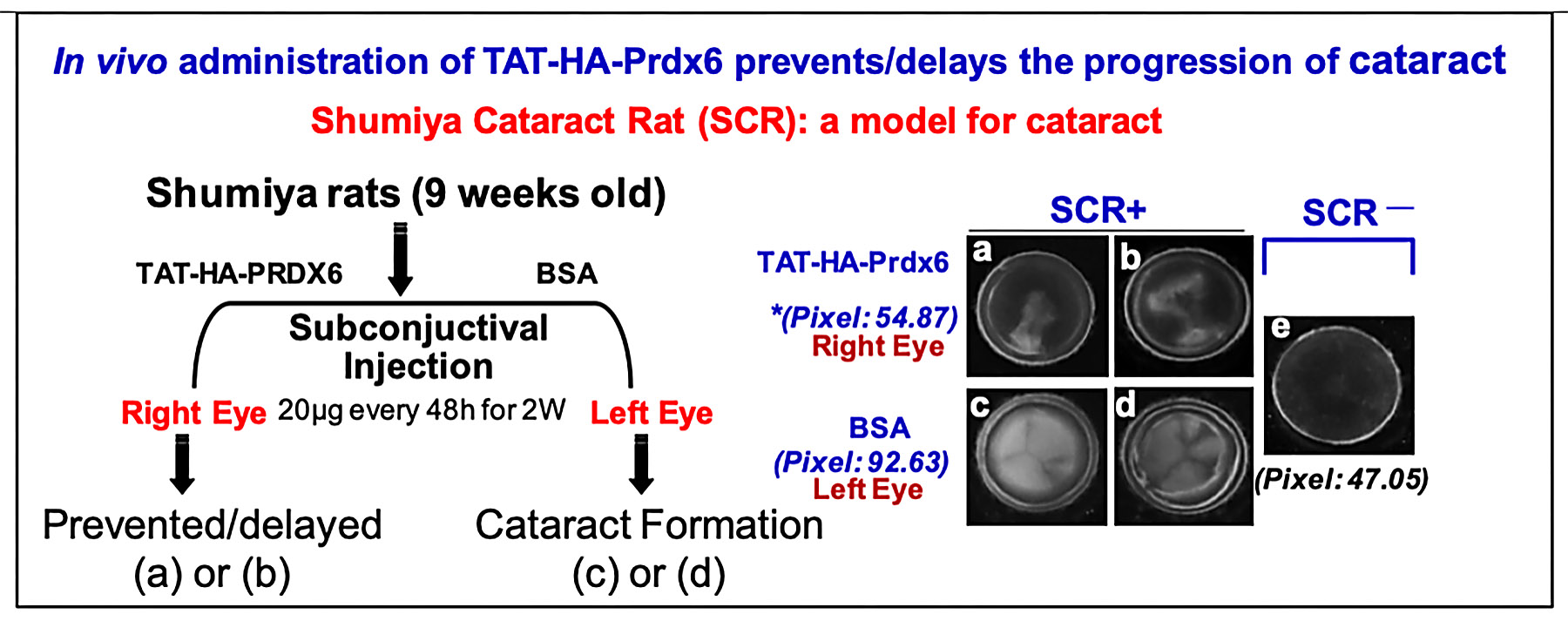
Recent innovative research has studied delivery to the eye lens of Prdx6 linked to TAT transduction domain. Using the Shumiya cataract rat (SCR), a model for hereditary cataract, Singh and colleagues have shown for the first time that recombinant Prdx6 linked to TAT, when injected subconjunctivally, can reach the lens, internalize into LECs, and delay cataractogenesis (figure below). Singh has expanded this research to diabetic complications and glaucoma. In collaborative studies, his group found that Prdx6 protected LECs against hyperglycemia-induced toxicity, and that a supply of Prdx6 attenuated environmental and cellular stresses that evoke cellular abnormalities, thereby protecting ganglion and trabecular-meshwork cells. This research holds great promise for treatments of blinding diseases, particularly age-related cataract and diabetes, possibly suggesting nonsurgical means of treating these conditions, which are the leading causes of blindness in the world today.
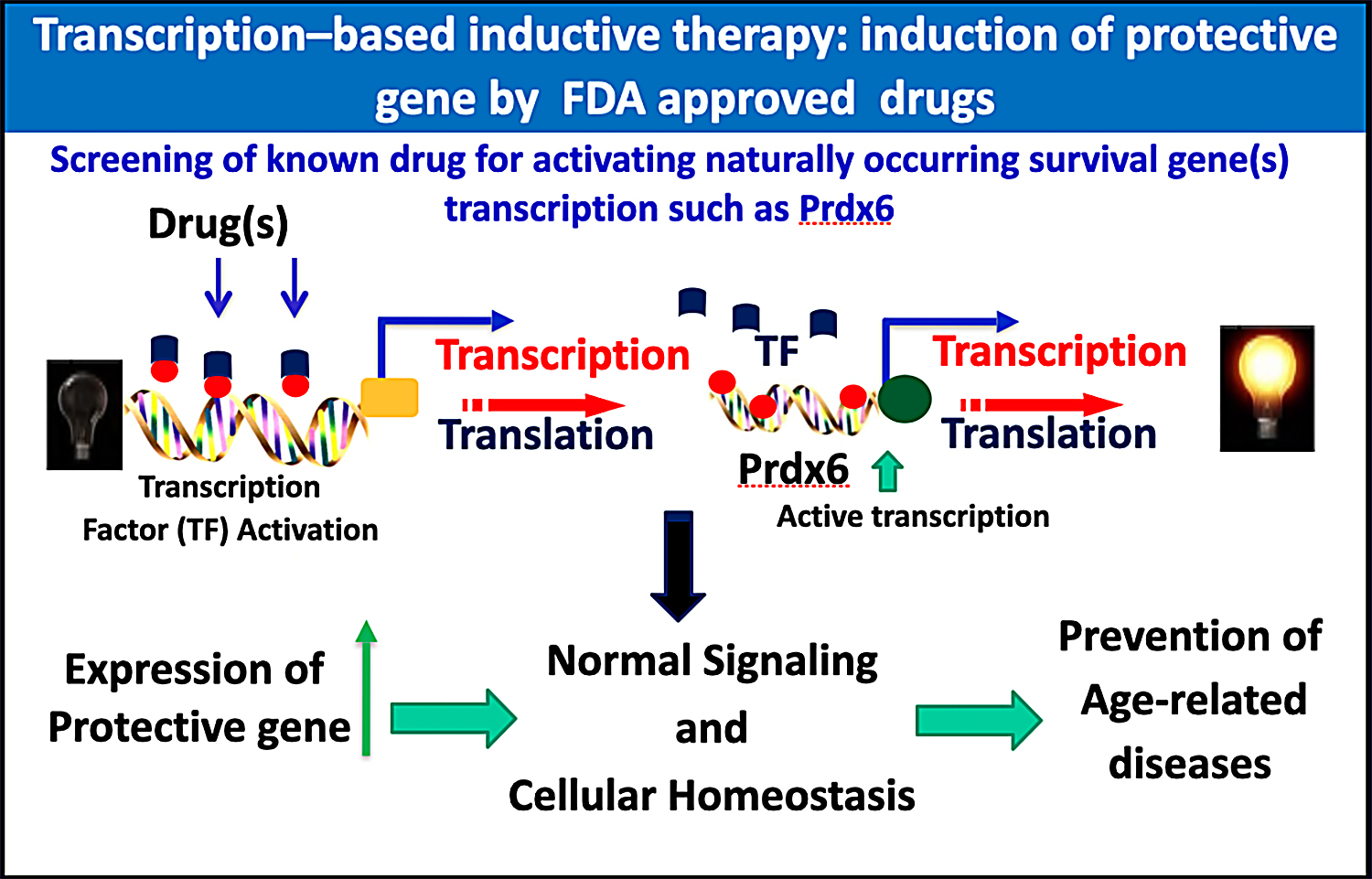
Recently, Singh and his group demonstrated that Prdx6 is multifunctional protective protein and saves many cell types from cellular stressors. More recently Singh team has become interested in more mechanistic studies of Prdx6’s functions and its regulation at the transcription level to develop transcription-based therapy for inducing natural cellular defenses by means of supplying dietary supplements. Considering the difficulty of protein delivery, this would be a novel approach to identify dietary compound(s) that may enhance the body’s antioxidant defenses. Along this line, he and his team are determining downstream signaling involved in regulation of Prdx6 transcription by using dietary supplements as well as FDA-approved drugs. His group has identified such compounds, and are now examining their efficacy in vivo. The strategy of using dietary supplements or existing drugs, if successful, could be employed widely to attenuate oxidative stress-associated degenerative diseases including ocular disorders.
The current research is interesting in its extension of the idea of developing transcription-based stimulation of an endogenous molecule to protect cells against stressors, through the use of dietary supplements (inductive therapy) (figure below).