Anna Dunaevsky, PhD, Lab
Dr. Anna Dunaevsky’s research is focused on how brain circuits are formed during development and how they are altered with learning. This is done by studying how the synaptic connections between brain cells change as a motor skill is learned. Since difficulties with motor skill learning are common in children with autism and other developmental disorders, her studies of the synaptic mechanism of motor skill learning are very relevant to these disorders. State-of-the-art microscopy approaches are used to follow the same neurons and synapses at different stages of development and learning in mice. Understanding the neurological mechanisms underlying the learning process is expected to provide important knowledge to develop therapies for cognitive disabilities and autism spectrum disorders.
Synaptic development in mouse models of neurodevelopmental disorders
Synapses are specialized sites of contact between cells that are critical for neuronal communication. Information is thought to be stored in the brain by creating, remodeling and modifying the effectiveness of synapses. Research in our lab focuses on understanding the cellular and molecular mechanisms that underlie the formation, maintenance and modification of synapses in the central nervous system. Specifically, we focus on small structures called dendritic spines that are the sites of majority of the excitatory synaptic input in the CNS. Proper dendritic spine structure, motility and organization are likely critical for synaptic efficacy while abnormalities may be related to intellectual disabilities associated with conditions such as Down, autism and fragile-X syndromes. Thus, identifying the mechanisms that regulate the structural features of spines is important for understanding elements of synaptic plasticity, cognitive function and mental disease.
Current Studies
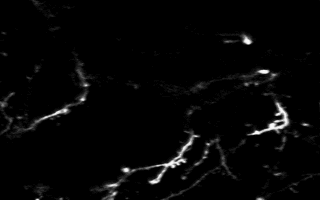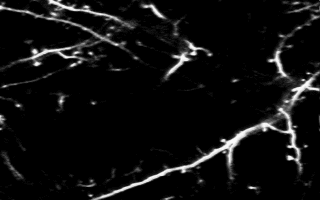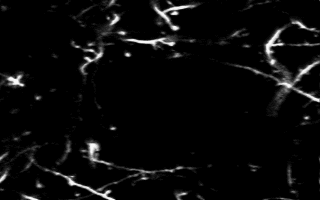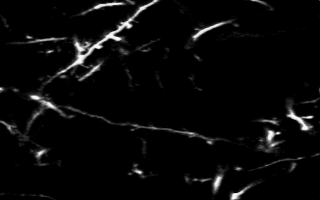
Multiphoton in vivo imaging in a YFP-H mouse
Fragile X Syndrome
The most common form of inherited intellectual disability is caused by loss of expression of the fragile X mental retardation protein (FMRP). We use the fmr1 null mouse to study how the functional and structural plasticity of synapses is altered in this disorder.

Neuron-glia Interactions
Recently, astrocytes have been shown to play an active role in regulating synaptic development and function and have been implicated in neurodevelopmental disorders. In another project we are investigating the role of cortical astrocyte calcium signaling in regulating dendritic spine morphogenesis and stabilization during development and learning.
We are also examining the contribution of astrocytes to Fragile X syndrome by studying how loss of FMRP in astrocytes contributes to neuronal impairment and sensory hypersensitivity. We are performing these experiments in mice as well as in astrocytes derived from FXS patient human induced Pluripotent Stem Cells (hiPSC).
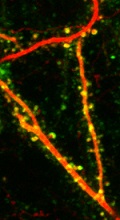
AMPA receptor trafficking is monitored in vivo with SEP-GluA expression
Experience dependent synaptic plasticity
Experience-dependent regulation of synaptic strength has long been hypothesized to be the physiological basis of learning and memory. In addition to our developmental projects, we study the cellular and synaptic mechanisms of learning-induced plasticity in the motor cortex. Following motor skill training we employ in vivo imaging, electrophysiology and molecular approaches to determine learning induced changes in synaptic structure, function and molecular composition and impairments in FXS mouse models.
Maternal immune activation (MIA)
Although the role of genetics in neurodevelopmental disorders such as autism spectrum disorder (ASD) is indisputable, increasing evidence points to environmental factors. MIA is an environmental risk factor for both autism and schizophrenia. We have been studying how behavior as well as synaptic structure and function is affected in MIA offspring. Ongoing studies examine the molecular changes that underlie these impairments as well as interactions between MIA and genetic risk factors.
Approaches used in the Lab
- Mouse behavior
- Two-photon microscopy (slice and in vivo imaging)
- Slice electrophysiology
- Molecular biology (In utero electroporation, viral transduction)
- Human Induced Pluripotent Stem Cells
- Histology (immunohistochemistry, electron microscopy)
Lab Members



Zahra Arbabi
Research Technologist I

Niveditha Sankar
Graduate Student - Neuroscience Program
