Lynda Harris, PhD - Lab

Harris Lab Members




Research Interests
- Pregnancy
- Placental function
- Obstetric nanomedicines
- Maternal and fetal health
Placental Drug Delivery
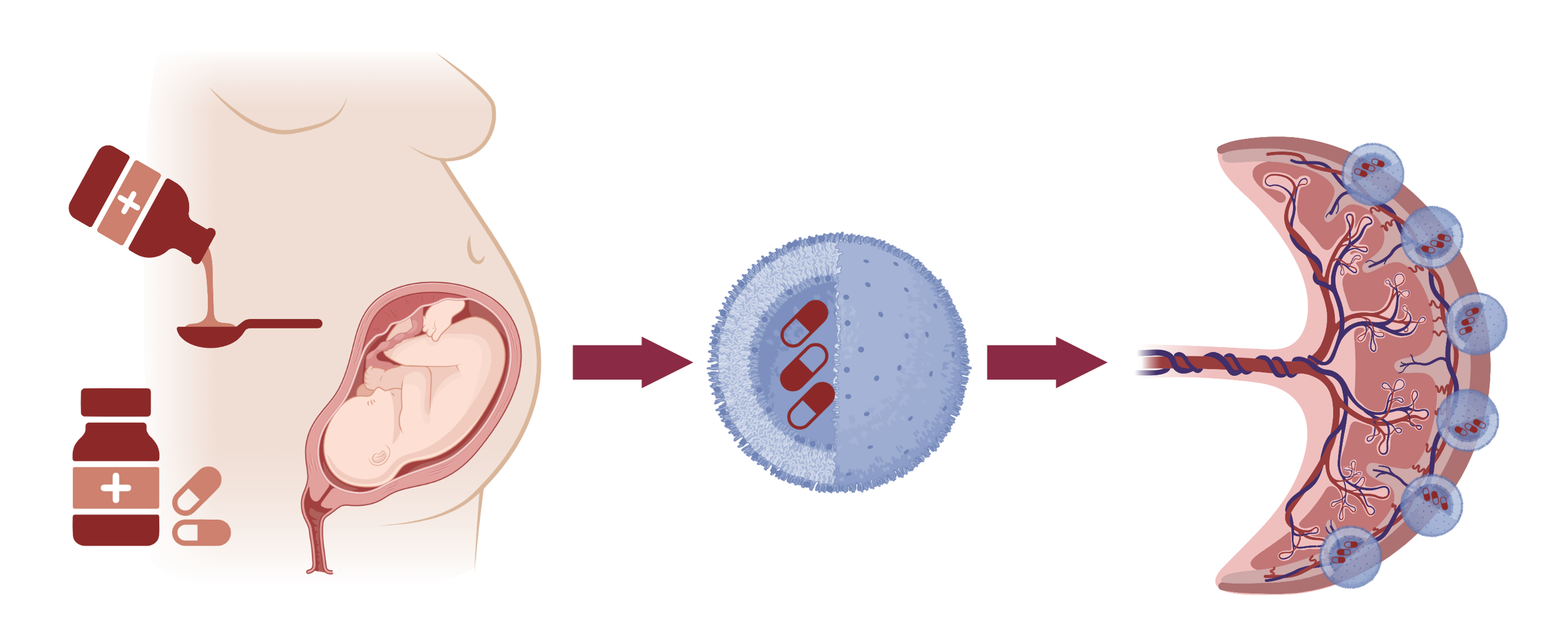
To reduce the risks associated with taking medicines in pregnancy, we have created targeted nanoparticles decorated with placental homing peptides, which can selectively deliver drugs to the placenta. This approach prevents fetal drug transfer and has enabled us to create the first therapeutics specifically designed to treat placental dysfunction. Image created with BioRender.
Identification of Novel Peptides that Bind to the Mouse Placenta
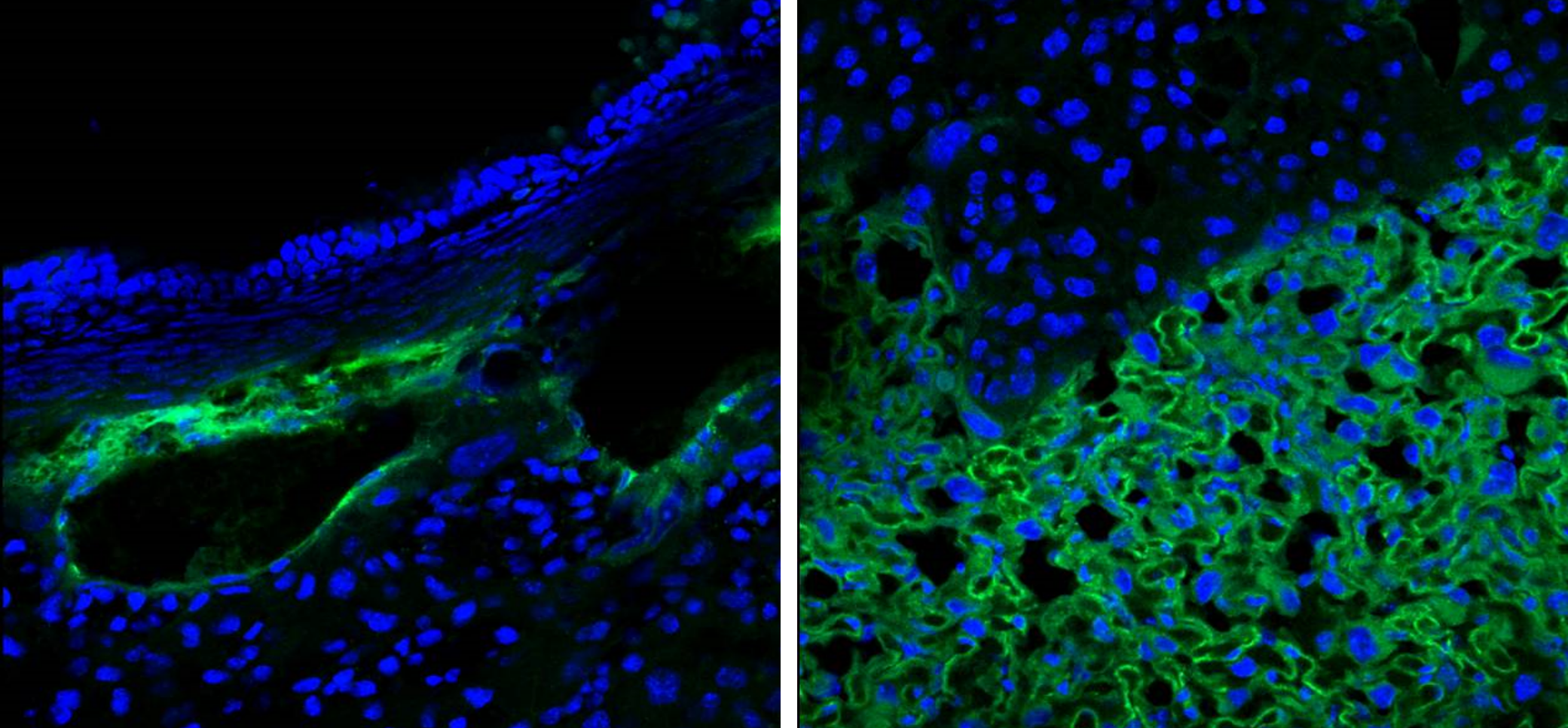
Fluorescently labeled peptide (green) was intravenously injected into a pregnant mouse (embryonic day 17.5) and allowed to circulate for 3 hours. Following cardiac perfusion with saline to remove unbound peptide, peptide binding was visualized by confocal microscopy. Homing peptide binding to mouse placental blood vessels (left) and the placental labyrinth (right) was observed. Nuclei are stained with DAPI (blue).
Research Projects
In pregnancy, the placenta is the key regulator of normal fetal growth and development. In the Western world, placental dysfunction is a major underlying cause of the pregnancy complications pre-eclampsia, fetal growth restriction and stillbirth. The term “placental dysfunction” is used to describe a range of anatomical, physiological, metabolic and endocrine alterations, which can occur in isolation, or in combination. Thus, to develop effective medicines to treat placental dysfunction, we need to identify a range of different compounds to treat the different phenotypes of placental dysfunction observed. For example, some placentas do not grow adequately, and have a small surface area for nutrient exchange. Others may be of an appropriate size, but their blood vessels may not function correctly, resulting in abnormal delivery of blood (and therefore nutrients and oxygen) to the developing baby. Others may show signs of immune cell infiltration, inflammation or oxidative damage, which can affect normal cell signaling pathways, cell metabolism and production of key hormones, growth factors and cytokines. Ongoing studies are working to identify drug compounds that are can be used to treat these different aspects of placental dysfunction, but that are also safe for use in pregnancy.
A major reason for the lack of medicines available to treat pregnancy complications is because of the potential risk of causing harm to the developing baby. As most medicines can cross the placenta and reach the fetal circulation, the pharmaceutical industry often exclude pregnant individuals from clinical trials, for fear of drugs causing developmental abnormalities, deformities, or other side effects in the fetus. Because of this, we lack important safety data for many medicines in pregnancy, and no specific medicines exist to treat pregnancy complications or placental dysfunction. This leaves medical professionals reliant on patient monitoring, treating symptoms e.g. high blood pressure, and early delivery of the baby if its condition deteriorates. To address this issue, my laboratory has identified a series of placental homing peptides, which selectively bind to the surface of the placenta in mice, non-human primates and humans, but do not accumulate in any other organs. We have used these peptides to develop biocompatible, targeted nanoparticles to selectively deliver drugs to the placenta, preventing fetal drug transfer and reducing drug effects in the maternal circulation. Ongoing studies are optimizing nanoparticle design, quantifying the benefits of targeted drug delivery versus systemic drug delivery, and collecting important safety data.
MicroRNAs (miRNAs) are short, non-coding RNA sequences that regulate multiple downstream signaling pathways. Placental growth and function are regulated by a variety of miRNAs, both in healthy pregnancy and in fetal growth restriction (FGR). We have used our novel placental homing peptides to create homing peptide-miRNA inhibitor conjugates, and demonstrated their ability to promote growth signaling in human placental explants and in a mouse model of FGR. These conjugates are made from synthetic peptide nucleic acids, which cannot be degraded in the human body and are therefore inherently stable. The addition of a placental homing peptide ensures that the conjugates are selectively delivered to the placental surface, where they spontaneously internalize into placental cells, overcoming previous problems associated with transfection of DNA or RNA-based gene therapies. Our current work aims to identify miRNAs which regulate key aspects of placental function and can be exploited as therapeutic targets. Homing peptide-miRNA mimetics or inhibitors can then be created to manipulate these miRNAs, improving placental function by targeted manipulation of placental gene expression. This work addresses the urgent need for safe and effective small molecule interventions to treat placental dysfunction and FGR.
Grants
1. NIH R01: Strategies to define and mitigate the placental and fetal alterations caused by maternal oxycodone exposure.
PI: LK Harris, Co-PI: G Pendyala. Co-Is: E Peeples, JS Davis
08/2023 – 07/2026
2. Mary G. and George W. White Fund for Medical Research: Transforming gynecological drug delivery by exploiting ovarian homing peptides.
PI: LK Harris. Co-Is: SY Kim, J Santarpia.
07/2023 – 06/2025
3. Olson Center for Women’s Health pilot grant: Assessing the effects of dolutegravir on placental development.
PI: A Bade; Co-I: LK Harris.
07/2023 – 06/2025
Former Lab Members
- Ayla Young, SURP student, Summer 2024
- Jill Ziegenbein, Medical Student, Summer 2024
- Megan Goeckel, Graduate Research Assistant, 2023-2024
- Guojuan Li, Research Technologist I, 2023-2024
- Claire Wing, SURP student, Summer 2023

Harris Lab, Summer 2023

Harris Lab, Summer 2023

Dr. Harris presents at the OB-GYN Grand Rounds, October 2023
Former SURP student Claire Wing presents her research poster, August 2023