Federal agencies continue to evolve the disclosure requirements designed to mitigate improper influence in federally funded research, with particular attention to disclosures regarding foreign involvement. Currently, the changes below apply only to the National Institutes of Health.
Important Changes
On 12 March 2021, the National Institutes of Health (NIH) issued a notice which set forth new requirements in the form of updated, reorganized forms and instructions for the Biosketch and Other Support documents. The changes are substantial in order to promote transparency and full disclosure of all resources—both domestic and foreign—supporting an individual’s research endeavors. The most dramatic new requirement is a certifying signature by the PD/PI or other senior/key personnel on their own Other Support form.
Signature Block Added: All PD/PIs and other senior/key personnel must electronically sign the Other Support form, prior to submission to NIH, certifying that the information is accurate and complete.
Guidance for Review
All PIs and all Department Administrators involved in research administration should review Notice No. NOT-OD-21-110 and Notice No. NOT-OD-21-073, along with the documents below, to begin understanding the new requirements:
- Biosketch: Instructions and FAQs
- Other Support: Instructions and FAQs
- Drop In Sessions (1st week of May): Presentation Slides with Resources
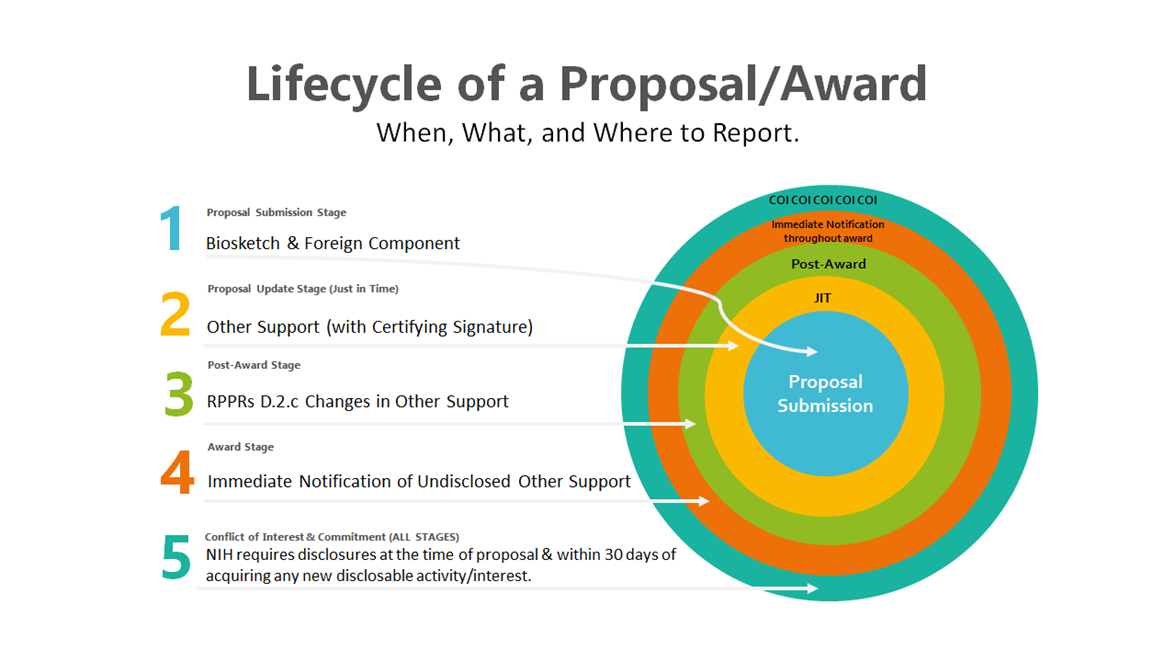
All Stages of Lifecycle
The new requirements affect all stages of the NIH proposal and award lifecycle:
- Proposal Submission Stage
- Just-in-Time (Proposal Update) Stage
- Post-Award Stage:
- RPPRs
- Immediate Notification of Undisclosed Other Support
If UNMC discovers that a PD/PI (or other senior/key personnel on an active NIH grant) failed to disclose Other Support information outside of Just-in-Time or RPPR submissions, the institution must submit updated Other Support to the Grants Management Specialist named in the Notice of Award immediately.
- Conflict of Interest (ALL STAGES)
Effective for Submissions to NIH Due Dates on or after 25 January 2022
- Proposals: All proposals submitted to the NIH with Due Dates on or after 25 January 2022 must use the reformated Biosketch.
- JITs & RPPRs: All Biosketch and Other Support documents submitted to the NIH on or after 25 January 2022 must use the reformattted Other Support and Biosketch.

