Thoreson Lab

Research interests and techniques
Dr. Thoreson’s laboratory focuses on mechanisms of visual information processing by the retina and particularly how rod and cone photoreceptors transmit visual information to second-order retinal neurons known as horizontal and bipolar cells. Rods and cones differ from many other nerve cells in that they do not produce sodium-dependent action potentials but instead respond to light with graded changes in membrane potential. These light-evoked voltage changes modulate calcium influx through L-type calcium channels that in turn control release of the neurotransmitter, glutamate, from photoreceptor terminals. Release of glutamate-filled synaptic vesicles from photoreceptor terminals involves plate-like protein structures known as synaptic ribbons.
To study neurotransmission in the retina, we employ a variety of different electrophysiological and imaging techniques. Electrophysiological studies involve single channel and whole cell patch clamp, capacitance measurement of exocytosis, flash photolysis of caged calcium compounds, and simultaneous paired whole cell recordings (e.g., rod-horizontal cell or cone-bipolar cell pairs) from photoreceptors and second order neurons. Preparations include wholemount retinas, retinal slices and solitary cells from both amphibian and mammalian retina.
Imaging studies have used spinning disk and two photon confocal microscopy along with ion-sensitive dyes such as the calcium-sensitive dyes Fluo-4 and Fura-2 and genetically encoded dyes such as iGluSnFr. We also developed techniques to visualize individual synaptic vesicle fusion events using total internal reflectance microscopy. Dye-loaded vesicles abruptly disappear as they fuse and release their contents. We have used single particle tracking techniques to study the membrane movements of individual calcium channels labeled with brightly fluorescent quantum dots.
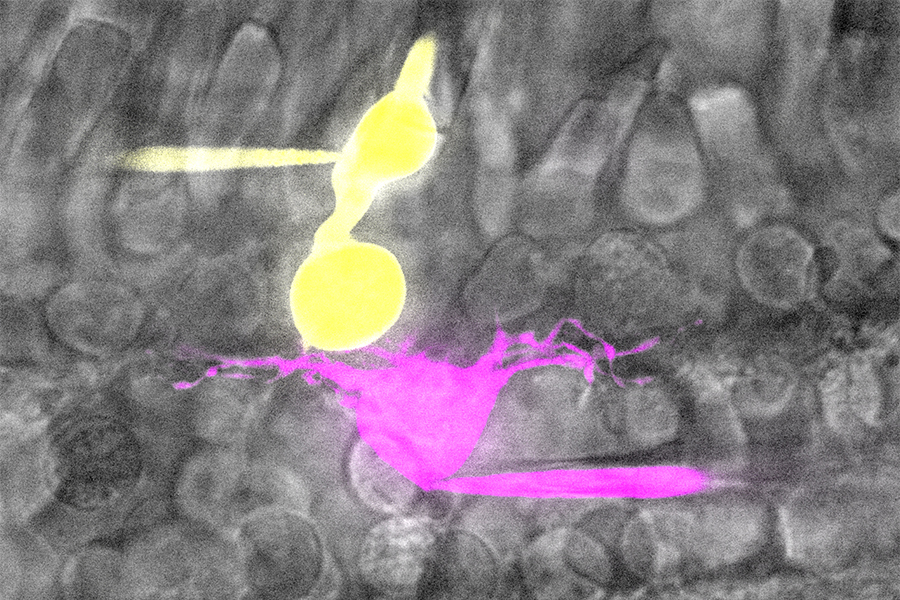
Cone (yellow) and horizontal cell (magenta) in salamander retina filled with Lucifer yellow (yellow) and Sulfarhodamine B (magenta) through glass pipette. Imaged with spinning disk confocal microscope.
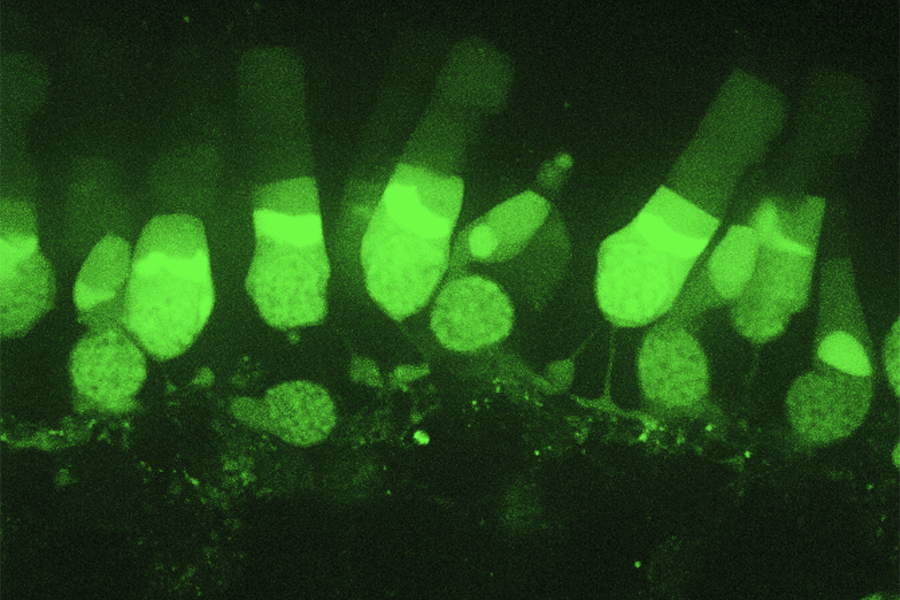
Photoreceptor cells (rods and cones) labeled with calcium sensitive dye Fluo4-AM in salamander retina. Imaged with spinning disk confocal microscope.
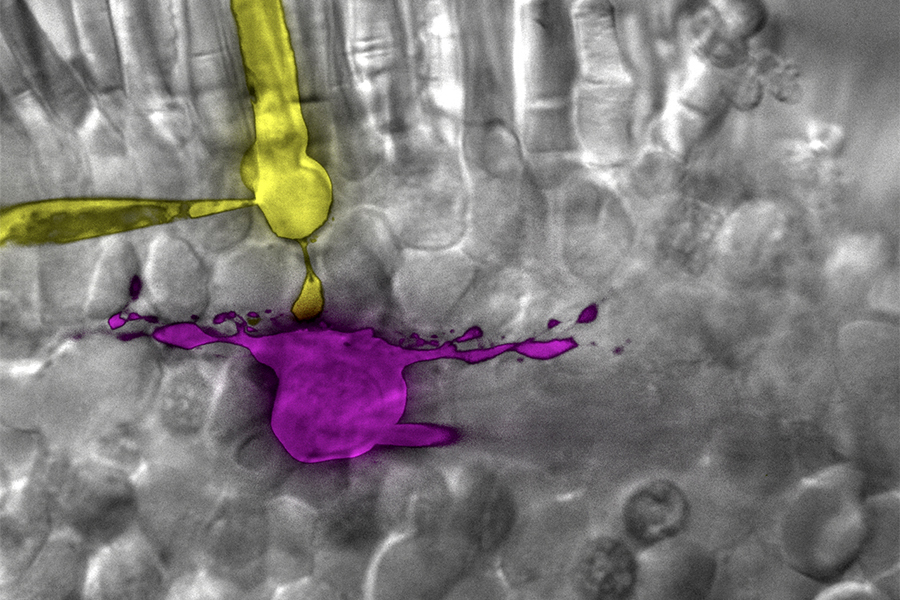
Rod (yellow) and horizontal cell (magenta) in salamander retina filled with Lucifer yellow (yellow) and Sulfarhodamine B (magenta) through glass pipette. Imaged with spinning disk confocal microscope.
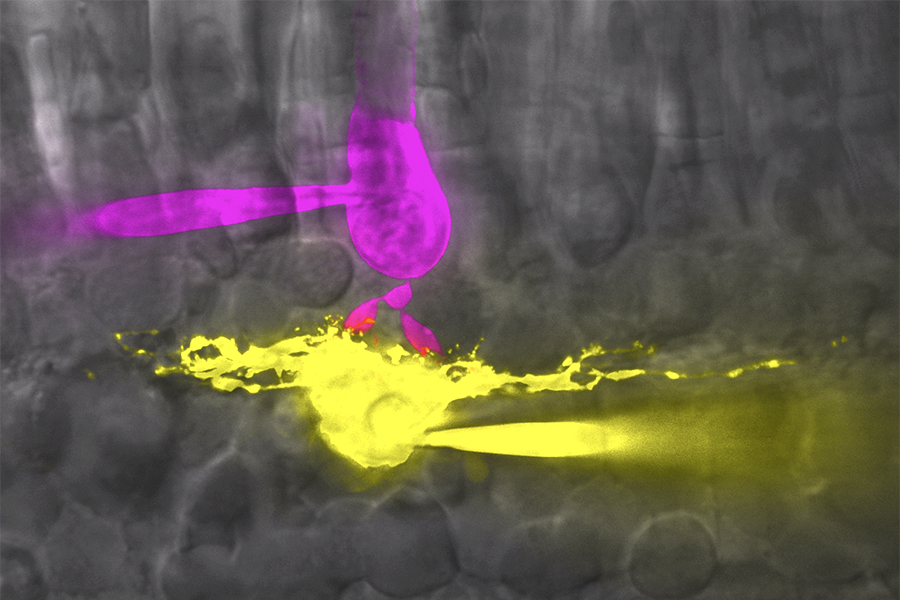
Rod (magenta) and horizontal cell (yellow) in salamander retina filled with Lucifer yellow (yellow) and Sulfarhodamine B (magenta) through glass pipette. Imaged with spinning disk confocal microscope.
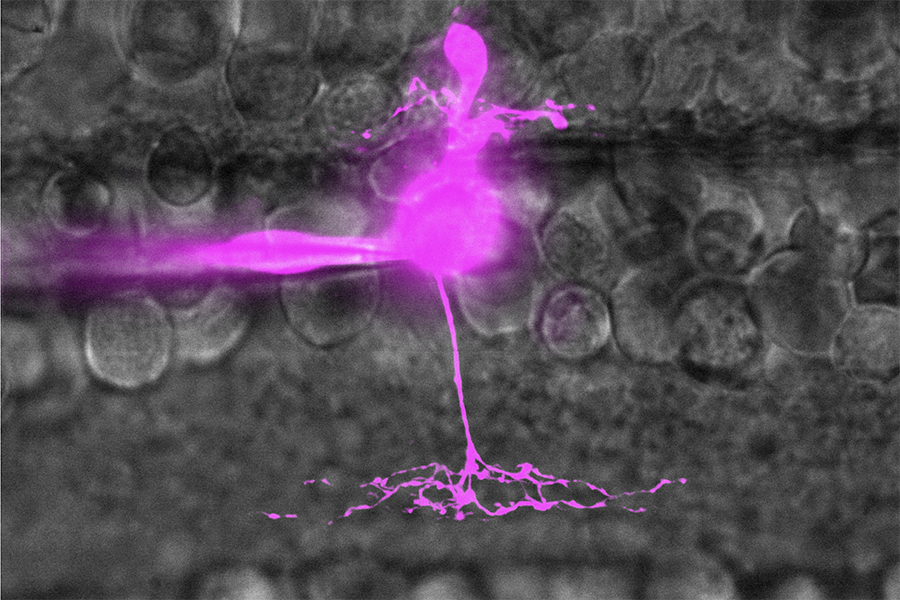
On type bipolar cell in salamander retina filled with Sulfarhodamine B (magenta) through glass pipette. Imaged with spinning disk confocal microscope.
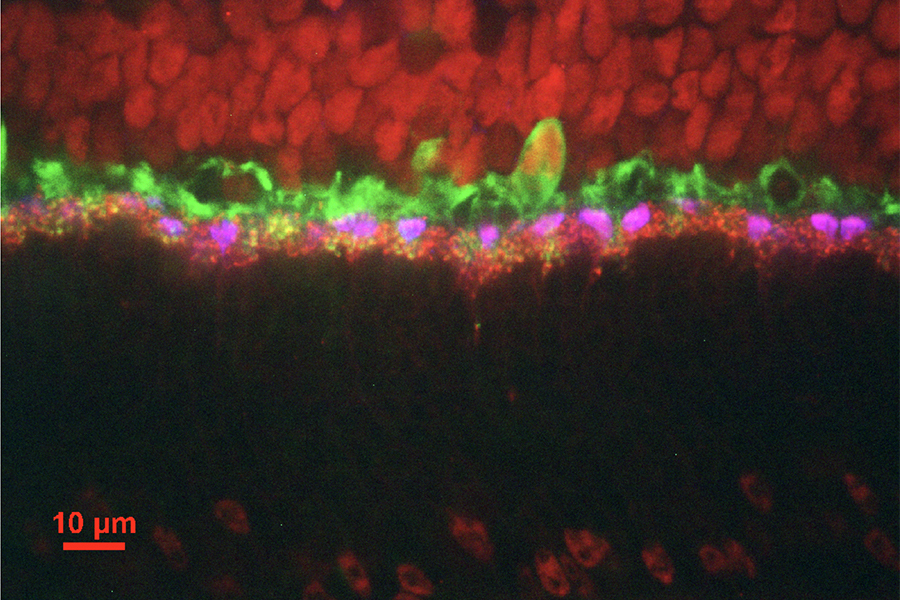
Mouse retina stained with labels for three different proteins (CtBP2, red; Syt1, magenta; calbindin, green). Imaged with spinning disk confocal microscope.
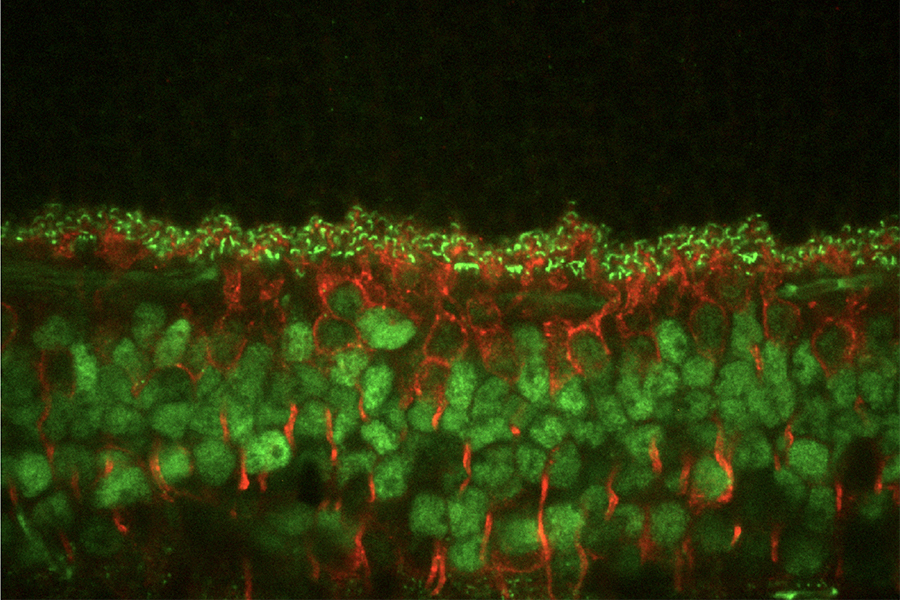
Mouse retina stained labels for two different proteins (green: CtBP2; red: PKCa). Imaged with spinning disk confocal microscope.
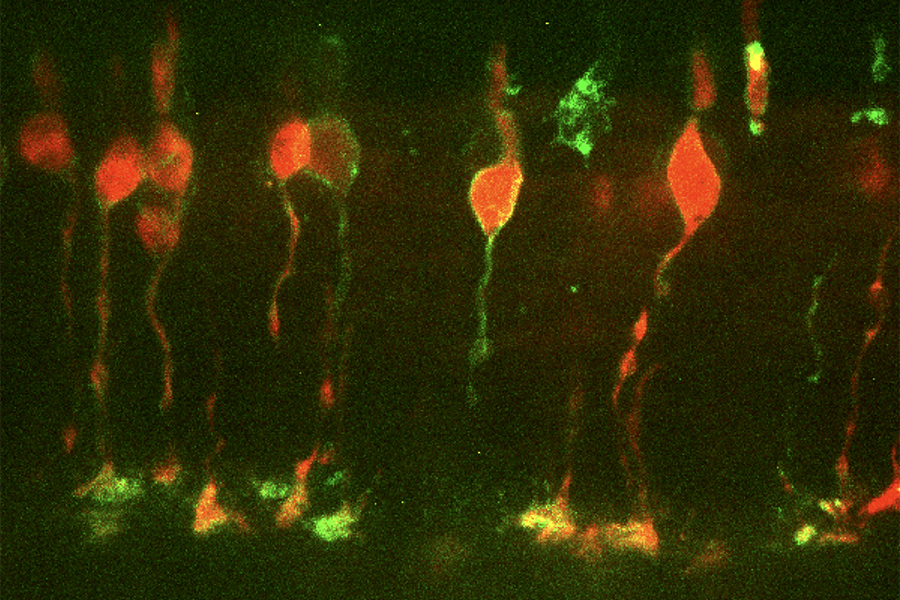
Cone photoreceptor cells in mouse retina expressing channelrhodopsin2 (green) in the membrane and td-tomato (red) in the cytoplasm. Imaged with spinning disk confocal microscope.
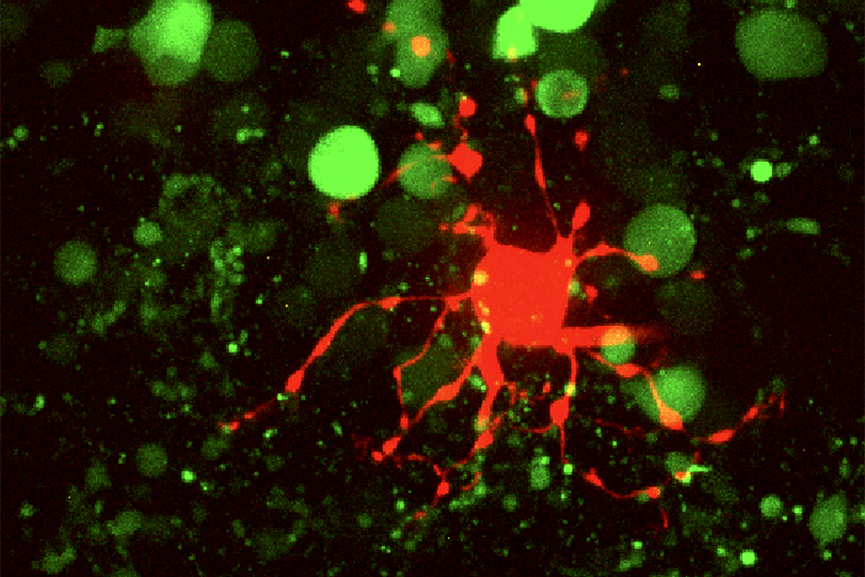
Horizontal cell (red) in mouse retina filled with Sulfarhodamine B (magenta) through glass pipette. Green: Cal520 dye in cone photoreceptor cells. Imaged with spinning disk confocal microscope.
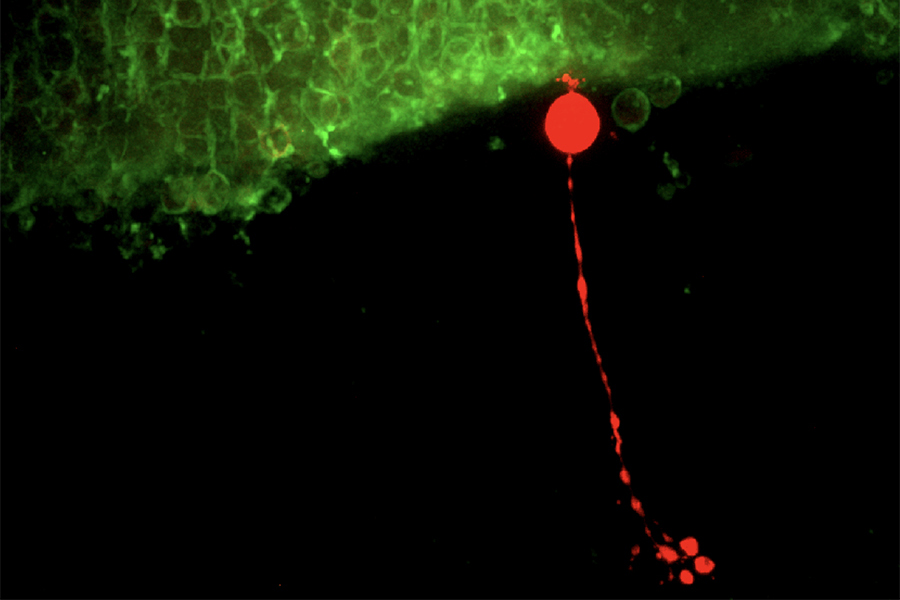
Rod bipolar cell filled with fluorescent dye below rods expressing ChannelRhodopsin2 used for optogenetic stimulation - living mouse retina.

Wallace B. Thoreson, PhD
Gilmore Professor, UNMC Department of Ophthalmology and Visual Sciences
Vice Chair and Research Director, Stanley M. Truhlsen Eye Institute
Professor, UNMC Department of Pharmacology & Experimental Neuroscience
Professor, University of Nebraska at Omaha Department of Biology