 Timothy Greiner, MD My research involves the study of the etiology and progression of lymphoma by examining the mutational spectra of oncogenes and tumor suppresser genes. My laboratory has been involved in characterizing mutations in ATM, p53 and bcl-6. We are correlating mRNA expression patterns in lymphoma with patient survival, mutation status, and morphological subtypes. We are currently examining the methylation status of DNA in diffuse large cell and mantle cell lymphomas. The emphasis on molecular epidemiology involves the identification of EBV subtypes, the gene expression, and the molecular abnormalities in post-transplant lymphoproliferative disorders. (photo at left: Burkitt Lymphoma)
Timothy Greiner, MD My research involves the study of the etiology and progression of lymphoma by examining the mutational spectra of oncogenes and tumor suppresser genes. My laboratory has been involved in characterizing mutations in ATM, p53 and bcl-6. We are correlating mRNA expression patterns in lymphoma with patient survival, mutation status, and morphological subtypes. We are currently examining the methylation status of DNA in diffuse large cell and mantle cell lymphomas. The emphasis on molecular epidemiology involves the identification of EBV subtypes, the gene expression, and the molecular abnormalities in post-transplant lymphoproliferative disorders. (photo at left: Burkitt Lymphoma)
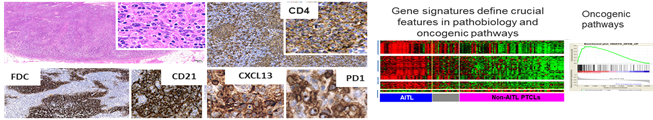
Javeed Iqbal, PhD The major focus of my lab is to understand the molecular mechanism of lymphomagenesis and to translate the relevant findings in lymphoma patient care. The lab extensively uses high-throughput genomic profiling techniques; including genome-wide mRNA, miRNA and DNA copy number analysis to improve molecular diagnosis and prognostication in lymphoid neoplasms. The long term research goal is genetic characterization and delineation of the molecular mechanisms leading to the transformation, in particular Peripheral T-cell lymphomas (PTCL). PTCL are poorly characterized lymphomas with aggressive clinical course and are challenging to study at the molecular level, due to lack of a reliable animal model and lymphoma cell lines. The lab has generated T-cell relevant mouse models to determine the cell intrinsic mechanisms of lymphomagenesis.