 Leah Cook, PhD
Leah Cook, PhD
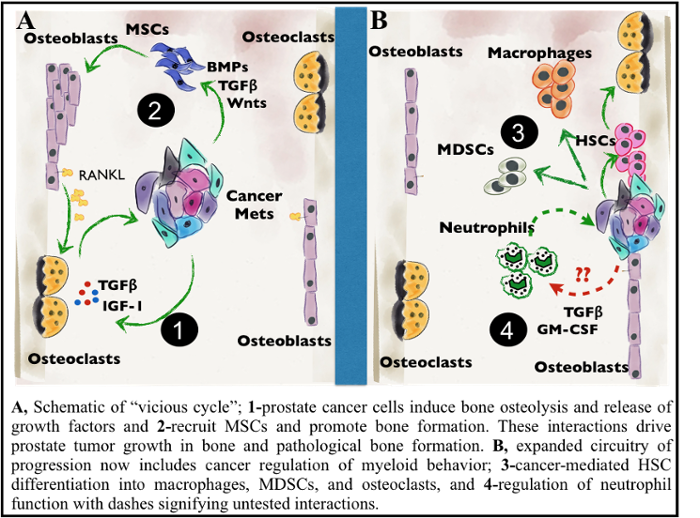
My lab’s research is focused on the role of the tumor microenvironment in cancer progression and metastasis. Prostate cancer metastasizes to bone more frequently than any other tissue site; metastatic progression to bone is associated with poor survival outcomes of prostate cancer. Within bone, metastatic cancer cells highjack the normal couple process of bone remodeling, resulting in excess bone degradation and subsequent release of growth factors that promote tumor growth (see figure). Additionally, cancer cells progress and mediate bone turnover through molecular and cellular interactions with the surrounding bone stroma. A major focus of the lab involves investigation of cellular interactions within the prostate tumor bone microenvironment that contribute to tumor progression and cancer-induced bone disease. We are currently focusing on identifying the importance of innate immune cells and bone stromal cells in prostate cancer progression and cancer-induced bone disease. Another focus of my lab is to define the importance of innate immune cells in metastatic progression and chemoresistance of pancreatic cancer. We are using a combination of transcriptome and proteomic profiling of patient samples and mouse in vivo models of cancer metastasis along with development of computational models to test putative targets. My goal is to identify novel immunotherapeutic targets for treating and curing metastatic cancers. Dr. Cook's research is also listed under Innate Immunity.
Rakesh K. Singh, PhD
The overall goal of our research is to define the mechanism(s) that regulate the process of metastasis. We hypothesize that metastasis is a highly selective process that is regulated by interrelated mechanisms whose outcome is dependent upon both the intrinsic properties of tumor cells and the host response. Using human tumors xenografted in nude mice and murine tumor models, these studies have demonstrated the role of host-derived factors in regulating angiogenesis, resulting in site-specific expression of angiogenic factors, including basic fibroblast growth factor (bFGF), interleukin-8 (IL-8), vascular endothelial growth factor (VEGF), and metastasis. Further characterization of the cellular and molecular mechanisms underlying these processes are currently ongoing in our laboratory. In addition, we are investigating the mechanism(s) of organ-specific metastasis. Recent reports suggest specific organ tissues carry unique marker molecules accessible to circulating cells. We have identified the molecule(s) expressed in organ tissues, which might be important to organ-specific metastasis using phage display libraries. Further characterization of organ-specific signature molecules will be useful in designing novel, highly targeted therapeutic approaches against organ-specific metastasis. In addition, our current research activities have also been focused on designing the strategies for inhibiting tumor-induced angiogenesis and activating anti-tumor immunity with the potential for synergizing the outcome of conventional therapeutic approaches, as well as understanding the role of tumor-stromal interaction in tumor progression and metastasis.
James Talmadge, PhD
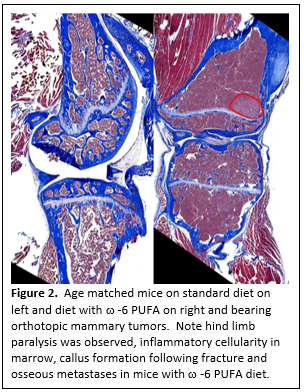
Basic/translational research studies are focused on host-tumor interactions during tumor progression, metastasis and cytoreductive therapy. We have focused on the effect of mammary tumor growth on the expansion and trafficking of MDSCs, and strategies to control proliferation and function, including molecular therapeutics. Our current focus is on dietary regulation in a collaboration with the Department of Genetics Cell Biology & Anatomy; Geoffrey Thiele, Internal Medicine; Timothy R. McGuire, Pharmacy; Leah Cook, Pathology/Microbiology; Paul Black, Dept. of Biochemistry UNL; and Concetta C. DiRusso, Dept. of Nutrition UNL. This collaboration builds on the observation that omega 3 and omega 6 poly unsaturated fatty acids (PUFA) regulate inflammation. Our studies have uniquely separated obesity from dietary PUFA using isocaloric and isolipidic diets. Our original studies reported PUFA regulation of hepatic and mammary gland histopathology that has been extended to assess the impact of dietary PUFA regulation of mammary tumor growth and metastasis. Perhaps the most exciting observation is the impact of omega 6 PUFA on accelerating tumor induction, growth and metastasis, including sites of metastasis that are depressed by omega 3 PUFA intervention. Dietary omega 6 PUFA not only increases pulmonary and hepatic metastases but also results in cardiac, contralateral mammary gland, ovarian and bone metastases (Figure 2). The latter is
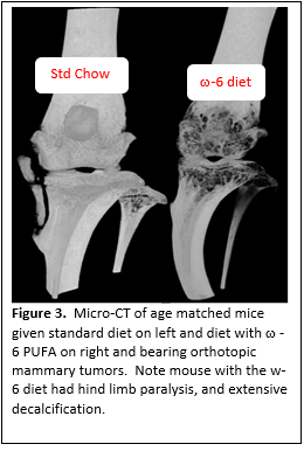
notable, as our metastasis model of orthrotropic primary tumors and spontaneous metastases to bone is unique, providing an ideal approach to study osseous metastasis. The overwhelming majority of bone metastasis studies uses models of artificial bone metastasis, via left ventricle injection or direct injection into or onto bones. Thus, in contrast to these approaches, our experimental approach incorporates all of the steps in the process of metastasis. The impetus for the studies into bone metastasis came from the observation of postural paralysis in tumor bearing mice on the omega 6 diets. In addition to Our studies to date have included gross, histologic and quantitative immunohistochemistry (IHC) analysis targeting neutrophils, macrophages, and T-cells, as well as proliferation and apoptosis. Mechanistic analysis has come from metabolomic, qRT-PCR and Western blots providing insight, not only in metastasis including osseous metastasis, but also prevention and interventional studies for neoplasia.histopathologic and immunohistochemical analysis, we have undertaken micro CT analysis that has revealed extensive decalcification (Figure 3). In association with the decalcification, we have observed spontaneous fractures (resulting in posterior paralysis) and callus formation. Dr. Talmadge's research is also listed under Transplantation Immunology.

