 Tammy Kielian, PhD
Tammy Kielian, PhD
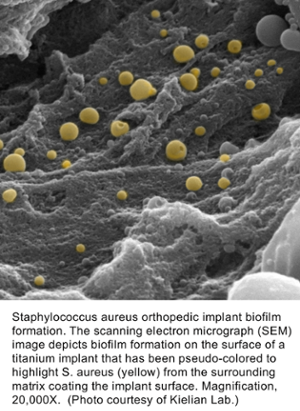
Dr. Kielian’s research interests span the fields of immunology, infectious diseases, and neuroscience with a unifying theme of innate immunity. Her laboratory has a long-standing interest in studying the pathogenesis and immune responses elicited by Staphylococcus aureus (S. aureus) both in the periphery and the central nervous system (CNS), with a particular emphasis on microglial and astrocyte activation. Dr. Kielian’s earlier work was focused on understanding neuroinflammatory pathways elicited during S. aureus brain abscess formation, which has transitioned to investigate immune mechanisms pertinent to S. aureus biofilm infections. To this end, her laboratory has developed mouse models of biofilm infection associated with orthopedic implants and cranial bone flaps that accurately mimic the attributes of biofilm infections in humans. Her laboratory was the first to propose that S. aureus biofilms actively elicit an anti-inflammatory immune signature to explain, in part, why these infections persist in an immune competent host. This is achieved by the preferential recruitment of myeloid-derived suppressor cells (MDSCs) in addition to polarizing macrophage infiltrates towards an anti-inflammatory, pro-fibrotic phenotype. Ongoing studies are to identify the mechanisms responsible for skewing the host innate immune response to an anti-inflammatory state following S. aureus biofilm infection and how this may be targeted to facilitate bacterial clearance. Her laboratory is utilizing high-throughput next-generation sequencing approaches (RNA-Seq and Tn-Seq) to elucidate critical molecules that promote biofilm development from both the host and bacterial perspectives. Other projects include 3D bioprinting approaches for the prevention/treatment of biofilm infections and active collaborations with orthopaedic surgeons and neurosurgeons at UNMC to investigate immune pathways in patients with prosthetic joint and craniotomy-associated infections, respectively. Dr. Kielian's research is also listed under Innate Immunity.
 Marilynn A. Larson, M.Sc., PhD
Marilynn A. Larson, M.Sc., PhD
Dr. Larson’s research is focused on elucidating the molecular mechanisms that allow intracellular pathogens to evade the immune system and persist. These assessments include the use of Francisella tularensis as a model system, since considerable differences in virulence exist between the subpopulations in this species, including select agents, attenuated strains, and avirulent strains. This facultative intracellular bacterium is the etiologic agent of the zoonotic disease tularemia, in which there is no effective vaccine. The highly infective strains are among the most pathogenic bacteria known with the potential to be used as a bioweapon and therefore, are classified as Tier 1 select agents by the Centers of Disease Control and Prevention. Although the genome of the type A.I, A.lI, and B clades within this species share 98% average nucleotide identity, insertion sequence elements have contributed to numerous chromosomal rearrangements between these subpopulations. The resulting changes in gene expression from these translocations, as well as other polymorphisms are being examined using a systems biology approach. These evaluations include the study of host-pathogen interactions elicited during a human macrophage infection. Together these investigations will provide a better understanding of the multifactorial mechanisms utilized by the highly virulent F. tularensis A.I strains, which enhances pathogenicity, and contribute to the development of effective countermeasures against F. tularensis and other intracellular pathogens. Additional research projects include the development of next generation molecular diagnostics for select agent and clinical pathogen identification and characterization, the detection of stable biomarkers that are indicative of an infection or radiation exposure, and the discovery of therapeutics to prevent or treat infections. Dr. Larson's research is also listed under High Risk Organisms and Host-pathogen Interactions.

