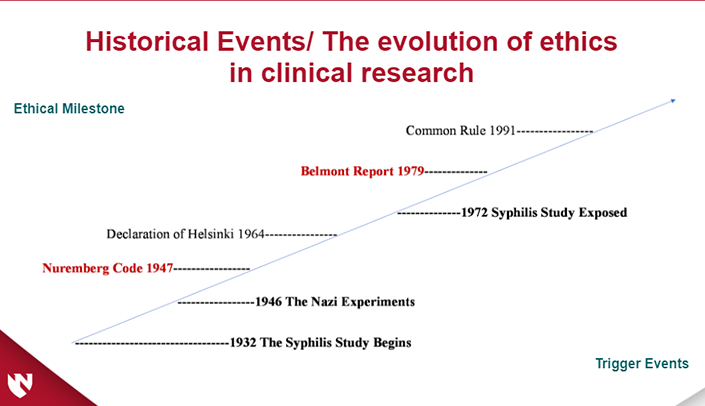
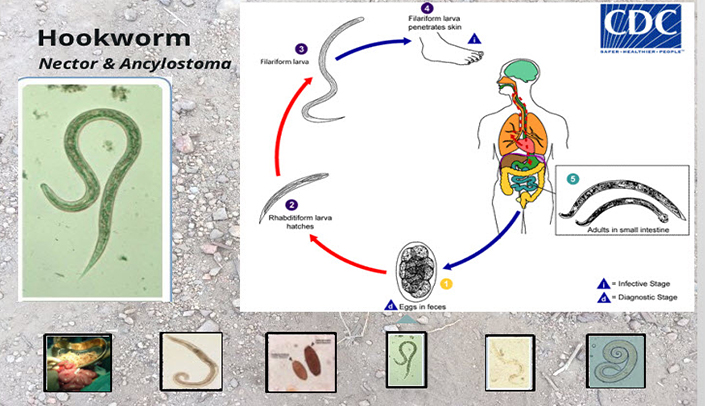
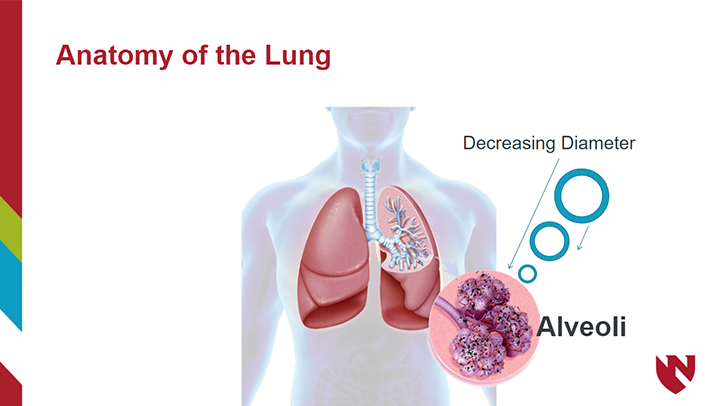
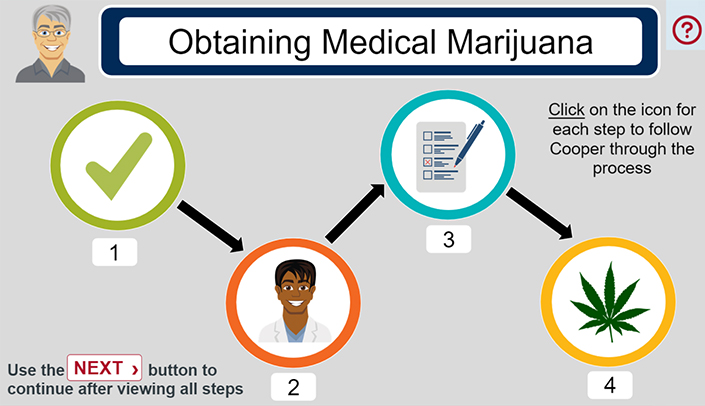
The module will cover the ethical perspectives of conducting clinical research. Recognize the historical events leading up to the Nuremberg Code and Belmont Report. Explain the three ethical principles in the Belmont report and eight ethical requirements.
Format: E-Learning Module
Development Date: April 12, 2021
Discipline: Public Health
Funding for the creation of this module was provided by an award from the Office of the Vice Chancellor for Academic Affairs at the University of Nebraska Medical Center
Permission: This content is available for faculty to use in their course. To show a link to this content, please complete the form below.