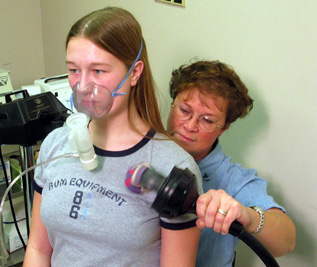 |
Kate Howard was diagnosed with cystic fibrosis when she was 5 months old. Here, her mom, Lonnel, assists Kate with an aerosol machine. Each morning and evening, they do about 50 minutes of treatments to loosen the thick, sticky mucus that clogs her lungs. Kate spends about 10 minutes on the machine and then about 40 minutes having her mother or father pound on her upper front and back for about 40 minutes to try to loosen the mucus. |
With cystic fibrosis, a defective gene causes the body to produce abnormally thick, sticky mucus that clogs the lungs and leads to chronic, life-threatening lung infections. These thick secretions also obstruct the pancreas, preventing digestive enzymes from reaching the intestines to help break down and absorb food. The chronic lung infections are responsible for shortening the life expectancy for CF patients to a median of approximately 33 years. In addition, many CF patients eventually develop diabetes.
“Cystic fibrosis is really a difficult disease to treat because so many organs are affected,” said John Colombo, M.D., professor and chief of the pediatric pulmonology section in the UNMC pediatrics department and a pediatric pulmonologist for The Nebraska Medical Center. “It’s a great honor for us to participate in this study. We’re excited to be able to help in the development of a potentially major new treatment for our CF patients. It is exciting because it is one of the first therapies actually targeted to treat the underlying defect.”
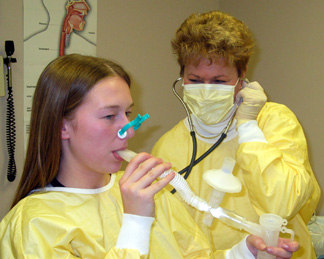 |
Nancy Hordvik, right, uses a nebulizer to turn the drug or placebo into an aerosol for patient Kate Howard. |
With CF patients, the CFTR gene has the wrong instructions and cells can’t make a CFTR protein that works properly. Without this protein, the lung airways can become blocked with mucous secretions, which can lead to severe, recurring respiratory infections.
Gene therapy, also called gene transfer, is an experimental procedure that is being developed to treat a variety of diseases, including CF, by putting normal copies of genes into cells that need them. Because genes are located inside cells, transferring new genes from the outside of a cell to the inside of a cell requires help.
In this study, a very small and simple virus, called AAV, has been engineered in the laboratory to contain a normal copy of the CFTR gene. AAV is not known to make people sick.
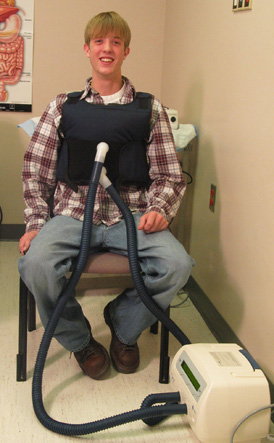 |
Chad Humston was diagnosed with cystic fibrosis about three months after he was born. Until six years ago, Chad underwent daily CF treatments in which his parents would put him on their lap and pound on his back to loosen the mucus. In 1998, he started wearing a special vest, which compresses his lung and back area to help loosen the mucus. Rhonda Humston calls the vest “a godsend.” |
Once the virus has infected the cells lining the bronchi (the branching airways within the lungs), the cell’s own machinery makes good CFTR from the good copies of the gene supplied by the virus and will continue to do so for the life of the cell.
Over the next several months, UNMC and The Nebraska Medical Center hopes to enroll about 10 patients into the study, Dr. Colombo said. The study provides for a total enrollment of 100 patients nationally. Two patients have already been enrolled at the medical center and have started receiving treatments.
Participants in the study will visit the CF center at the medical center eight times. During two of these visits, participants will receive tgAAVCF or a placebo. Participants will not know during the treatment if they are receiving the active drug or the placebo.
A nebulizer will be used to turn the drug or the placebo into an aerosol, which participants will breathe in through their mouths. By breathing in through their mouths, the drug or placebo will enter the airways in the lungs. Once the drug enters the airway, it enters cells.
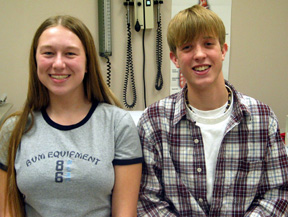 |
Kate Howard and Chad Humston are enrolled in the medical center’s cystic fibrosis study. |
Two patients enrolled in The Nebraska Medical Center/UNMC study are Kate Howard, 16, of Sioux City, Iowa, and Chad Humston, 17, of Giltner, Neb. Both participated in a news conference held at the Medical Center.
“Cystic fibrosis is a disease that reminds you that it’s there every day,” said Lonnel Howard, Kate’s mother. “Kate has never had one day of normal living. We are so excited to have Kate participating in this study. It represents hope for the future for all CF patients.”
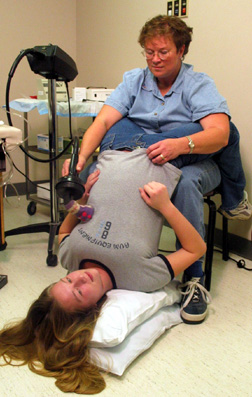 |
Part of Kate Howard’s daily treatment calls for Kate to lie upside down on her mother’s or father’s lap with her head resting on the floor, while they pound on her back. It’s another technique for loosening the mucus. |
The phase II study has now been published in the February issue of Chest, a leading scientific journal. It served as the basis for the current study, a Phase IIb study, which is being conducted by Targeted Genetics Corporation of Seattle, the company that manufactures the CF drug, and the Cystic Fibrosis Foundation.
“Gene therapy is one of the most exciting areas of scientific research,” Dr. Colombo said. “It is a potential mode of therapy for any disease with a genetic basis, so that can encompass lots of diseases, including several forms of cancer. The use of gene therapy in CF is probably the biggest breakthrough we’ve seen since the CF gene was discovered in 1989.”
 |
John Colombo, M.D., a pediatric pulmonologist, explains the clinical study during Thursday’s press conference. |
“We have seen a marked increase in the number of gene therapy protocols at UNMC in the last year,” Dr. Prentice said, “and we anticipate that we will continue to see an increase in gene therapy studies in the future.”
The CF gene therapy study is being administered nationally by Children’s Hospital and Regional Medical Center in Seattle. For more information on the study, call (402) 559-9256.